Lizard Development
| Embryology - 1 May 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
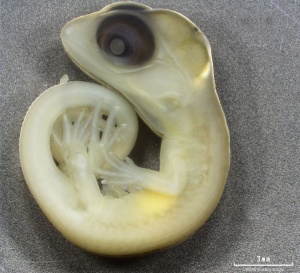
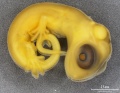
Lizards and snakes represent scaled reptiles (squamata). Lizard development involves an amniotic egg, that evolutionary (~320 million years ago) freed the vertebrates from their aquatic (water) to a terrestrial (land) environment. The Galápagos Islands marine iguana was also made famous by Charles Darwin's historic evolution studies.
The genome of the lizard Anolis carolinensis (green anole) from southeastern United States has a karyotype of 18 chromosomes, comprising six pairs of large macrochromosomes and 12 pairs of small microchromosomes, and has recently been sequenced[1]. Interestingly, almost all reptilian genomes also contain "microchromosomes", very small chromosomes less than 20 Mb in sequence size. (More? Genome)
| Lizard links: lizard | 1904 Sand Lizard | 1932 Twinning | Category:Lizard |
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Lizard Embryology | Lizard Development | Reptile Embryology |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Taxon
root; cellular organisms; Eukaryota; Opisthokonta; Metazoa; Eumetazoa; Bilateria; Coelomata; Deuterostomia; Chordata; Craniata; Vertebrata; Gnathostomata; Teleostomi; Euteleostomi; Sarcopterygii; Tetrapoda; Amniota; Sauropsida; Sauria; Lepidosauria
Squamata (squamates) - snakes and lizards.
- Iguania (iguanian lizards) - arboreal with primitively fleshy, non-prehensile tongues, highly modified in the chameleons.
- Acrodonta
- Iguanidae (iguanid lizards)
- Scleroglossa
- Amphisbaenia
- Anguimorpha (anguimorph lizards)
- Gekkota - all geckos and the limbless Pygopodidae.
- Scincomorpha (scincomorph lizards)
- Serpentes (snakes)
- unclassified Squamata
- Links: Taxonomy Browser Lizards
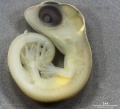
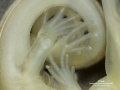
Development Overview
Australian Water Skink
Genome
Anolis carolinensis (green anole)
The genome of the lizard Anolis carolinensis (green anole) from southeastern United States has a karyotype of 18 chromosomes, comprising six pairs of large macrochromosomes and 12 pairs of small microchromosomes, and has recently been sequenced.[1] Interestingly, almost all reptilian genomes also contain "microchromosomes", very small chromosomes less than 20 Mb in sequence size.
It is a model organism for laboratory-based studies of organismal function and for field studies of ecology and evolution. This species was chosen for genome sequencing in part because of the ease and low expense of captive breeding, well studied brain, and sophisticated color vision. It is also well suited for studies involving the role of hormones in development and adult nervous system plasticity. (modified from Genome)
Search PubMed Genome: Lizard
References
- ↑ 1.0 1.1 Alföldi J, Di Palma F, Grabherr M, Williams C, Kong L, Mauceli E, Russell P, Lowe CB, Glor RE, Jaffe JD, Ray DA, Boissinot S, Shedlock AM, Botka C, Castoe TA, Colbourne JK, Fujita MK, Moreno RG, ten Hallers BF, Haussler D, Heger A, Heiman D, Janes DE, Johnson J, de Jong PJ, Koriabine MY, Lara M, Novick PA, Organ CL, Peach SE, Poe S, Pollock DD, de Queiroz K, Sanger T, Searle S, Smith JD, Smith Z, Swofford R, Turner-Maier J, Wade J, Young S, Zadissa A, Edwards SV, Glenn TC, Schneider CJ, Losos JB, Lander ES, Breen M, Ponting CP & Lindblad-Toh K. (2011). The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature , 477, 587-91. PMID: 21881562 DOI.
- ↑ Siviter H, Deeming DC & Wilkinson A. (2019). Egg incubation temperature influences the growth and foraging behaviour of juvenile lizards. Behav. Processes , 165, 9-13. PMID: 31170461 DOI.
- ↑ Ollonen J, Da Silva FO, Mahlow K & Di-Poï N. (2018). Skull Development, Ossification Pattern, and Adult Shape in the Emerging Lizard Model Organism Pogona vitticeps: A Comparative Analysis With Other Squamates. Front Physiol , 9, 278. PMID: 29643813 DOI.
- ↑ Stewart JR & Thompson MB. (2017). Yolk sac development in lizards (Lacertilia: Scincidae): New perspectives on the egg of amniotes. J. Morphol. , 278, 574-591. PMID: 28168721 DOI.
- ↑ Jensen B, Boukens BJ, Postma AV, Gunst QD, van den Hoff MJ, Moorman AF, Wang T & Christoffels VM. (2012). Identifying the evolutionary building blocks of the cardiac conduction system. PLoS ONE , 7, e44231. PMID: 22984480 DOI.
- ↑ Zahradnicek O, Horacek I & Tucker AS. (2012). Tooth development in a model reptile: functional and null generation teeth in the gecko Paroedura picta. J. Anat. , 221, 195-208. PMID: 22780101 DOI.
- ↑ Du WG, Ye H, Zhao B, Pizzatto L, Ji X & Shine R. (2011). Patterns of interspecific variation in the heart rates of embryonic reptiles. PLoS ONE , 6, e29027. PMID: 22174948 DOI.
- ↑ Gribbins KM. (2011). Reptilian spermatogenesis: A histological and ultrastructural perspective. Spermatogenesis , 1, 250-269. PMID: 22319673 DOI.
Reviews
Murphy BF & Thompson MB. (2011). A review of the evolution of viviparity in squamate reptiles: the past, present and future role of molecular biology and genomics. J. Comp. Physiol. B, Biochem. Syst. Environ. Physiol. , 181, 575-94. PMID: 21573966 DOI.
Dzialowski EM, Sirsat T, van der Sterren S & Villamor E. (2011). Prenatal cardiovascular shunts in amniotic vertebrates. Respir Physiol Neurobiol , 178, 66-74. PMID: 21513818 DOI.
Gamble T. (2010). A review of sex determining mechanisms in geckos (Gekkota: Squamata). Sex Dev , 4, 88-103. PMID: 20234154 DOI.
Articles
Lu HL, Wang J, Xu DD & Dang W. (2018). Maternal warming influences reproductive frequency, but not hatchling phenotypes in a multiple-clutched oviparous lizard. J. Therm. Biol. , 74, 303-310. PMID: 29801642 DOI.
Desfilis E, Abellán A, Sentandreu V & Medina L. (2018). Expression of regulatory genes in the embryonic brain of a lizard and implications for understanding pallial organization and evolution. J. Comp. Neurol. , 526, 166-202. PMID: 28891227 DOI.
Sanger TJ & Kircher BK. (2017). Model Clades Versus Model Species: Anolis Lizards as an Integrative Model of Anatomical Evolution. Methods Mol. Biol. , 1650, 285-297. PMID: 28809029 DOI.
Wise PA, Vickaryous MK & Russell AP. (2009). An embryonic staging table for in ovo development of Eublepharis macularius, the leopard gecko. Anat Rec (Hoboken) , 292, 1198-212. PMID: 19645023 DOI.
Noro M, Uejima A, Abe G, Manabe M & Tamura K. (2009). Normal developmental stages of the Madagascar ground gecko Paroedura pictus with special reference to limb morphogenesis. Dev. Dyn. , 238, 100-9. PMID: 19097047 DOI.
Storm MA & Angilletta MJ. (2007). Rapid assimilation of yolk enhances growth and development of lizard embryos from a cold environment. J. Exp. Biol. , 210, 3415-21. PMID: 17872995 DOI.
Fabrezi M, Abdala V & Oliver MI. (2007). Developmental basis of limb homology in lizards. Anat Rec (Hoboken) , 290, 900-12. PMID: 17415759 DOI.
Sanger TJ & Gibson-Brown JJ. (2004). The developmental bases of limb reduction and body elongation in squamates. Evolution , 58, 2103-6; discussion 2107-8. PMID: 15521466
Muthukkaruppan V, Kanakambika P, Manickavel V & Veeraraghavan K. (1970). Analysis of the development of the lizard, Calotes versicolor. I. A series of normal stages in the embryonic development. J. Morphol. , 130, 479-89. PMID: 5437480 DOI.
Mohammed MB. (1984). Development of the lizard limb as shown by the distribution of [35S]sulphate incorporation. J. Anat. , 138 ( Pt 3), 399-403. PMID: 6429113
Books
Herrick CJ. The Brain of the Tiger Salamander (1948) The University Of Chicago Press, Chicago, Illinois.
Search PubMed
Search PubMed: Lizard development | Anolis carolinensis
Historic
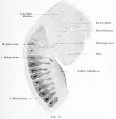
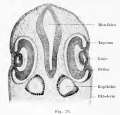
Schwalbe (1891) points out the significant fact that in reptiles that lack an external ear (lizard and turtle) there occur distinct hillocks in the embryo, resembling those in vertebrates that develop an auricle. These hillocks undergo degeneration and are reduced to the level of the surrounding skin. He finds in both birds and reptiles hillocks corresponding to the tragus and antitragus hillocks of His. These animals have one hillock (Auricularkegel), situated dorsal to the first cleft, which seems to represent a more primitive apparatus than is present in mammals, although it may be related to the helix system. In Salachians it possesses a spiracle.
(From Contributions to Embryology No.69)
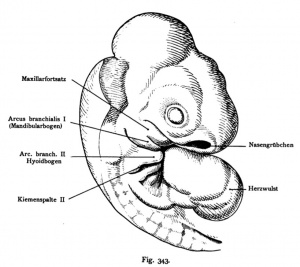
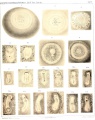
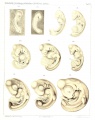
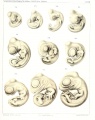
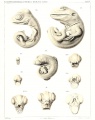
Sand Lizard 1904
Normal Plates of the Development of Vertebrates 4 - Sand Lizard (Lacerta agilis) by Karl Peter
The Brain of the Tiger Salamander 1948
Herrick CJ. The Brain of the Tiger Salamander (1948) The University Of Chicago Press, Chicago, Illinois.
Part I. General Description and Interpretation 1. Salamander Brains | 2. Form and Brain Subdivisions | 3. Histological Structure | 4. Regional Analysis | 5. Functional Analysis, Central and Peripheral | 6. Physiological Interpretations | VII. The Origin and Significance of Cerebral Cortex | VIII. General Principles of Morphogenesis Part 2. Survey of Internal Structure 9. Spinal Cord and Bulbo-spinal Junction | 10. Cranial Nerves | 11. Medulla Oblongata | 12. Cerebellum | 13. Isthmus | 14. Interpeduncular Nucleus | 15. Midbrain | 16. Optic and Visual-motor Systems | 17. Diencephalon | 18. Habenula and Connections | 19. Cerebral Hemispheres | 20. Systems of Fibers | 21. Commissures | Bibliography | Illustrations | salamander
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Department of Primary Industries Lizards of Tasmania
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, May 1) Embryology Lizard Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Lizard_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G