Bovine Development
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Bovine (taxon- Bos taurus) development is studied extensively due to the commercial applications of cattle both for milk and meat production. There is some variation in the gestation period (279-290 days) for the different breeds.
| Cattle Gestation Periods | (Bovine Development) |
|---|---|
| Breed | Average Days (±7–10 days) |
| Angus | 281 |
| Ayrshire | 279 |
| Brahman | 292 |
| Brown Swiss | 290 |
| Charolais | 289 |
| Guernsey | 283 |
| Hereford | 285 |
| Holstein | 279 |
| Jersey | 279 |
| Limousin | 289 |
| Shorthorn | 282 |
| Simmental | 289 |
| Bovine Links: Bovine Development | Category:Bovine | ||
|
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Bovine Embryology | Bovine Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Taxon
Bos taurus
Genbank common name: cow, bovine, domestic cattle
Taxonomy Id: 9913 Rank: species
Genetic code: Translation table 1 (Standard)
Mitochondrial genetic code: Translation table 2 (Vertebrate Mitochondrial)
Lineage( abbreviated ): Eukaryota; Metazoa; Chordata; Craniata; Vertebrata; Euteleostomi; Mammalia; Eutheria; Laurasiatheria; Cetartiodactyla; Ruminantia; Pecora; Bovidae; Bovinae; Bos; Bos taurus
Bovine Development
Implantation
Following implantation the conceptus secretes Interferon tau (IFNT) that is the signal for maternal recognition of pregnancy (Day 16). This maintains progesterone (P4) secretion and antagonizes the endometrial cells response to phorbol 12,13-dibutyrate (PDBU) an activator of protein kinase C.
|
The table below shows the general timing of early development stages in the bovine embryo, as well as comparing this to other domestic species. Implantation in the uterus occurs between 30-35 days.
(Data: Oklahoma State University Learning Reproduction in Farm Animals) |
|
General Overview
A historic general descriptive overview.[11]
- First month (28 days) - The embryonic period, the embryo is 9 to 10 mm long and the first signs of extremities appear.
- Second month (30 to 60 days) - The extremities develop. The pharyngeal cleft closes in the beginning of this month. The sternum still has a longitudinal fissure in the middle, closing toward the end of the eighth week. At the end of the second month at the end of each extremity are a little conical elevation, which is colorless and transparent. This is the first indication of the hoof. The length of the fetus is 48 mm In the ninth week its length is 8 cm.
- Third month (60 to 90 days) - Toward the end of this month the four stomachs may be recognized. The fetus measures 14 cm. in length. The scrotum is present.
- Fourth month (90 to 120 days) - In the beginning of the fourth month the hoofs become quite, distinct ; they are firm, non-transparent, and have a yellow color. The fetus is about 24 cm. long and weighs up to 2 kg. (Frauck).
- Fifth month (120 to 150 days) - In the beginning of the month the first tentaculse (tactile hairs) appear on the lips, chin, upper eyelid, and orbital arch. The teats are plainly visible. The testicles descend into the scrotum. The fetus, is about 35 cm. long and weighs 2.5 to 3 kg.
- Sixth month (150 to 180 days) - The eyelashes are more developed. The foetus is about 46 cm. long. The whole body is still naked excepting the lips and eyelids.
- Seventh month (180 to 210 days) - At the end of this month a few long hairs appear at the end of the tail; also hairs about the coronet and on the spots where the horns appear. The foetus is about 60 cm. long.
- Eighth month (210 to 240 days) - The back begins to be covered with hair, also along the edges of the ears. The length of the fetus toward the 32d week is 65 cm, and toward the end of this month 75 cm. (Franck).
- Ninth month - In the beginning the whole body is covered with hair and increases greatly in size. The fetus measures from 80 to 100 cm.
- Tenth month - beginning this month the fetus becomes mature.
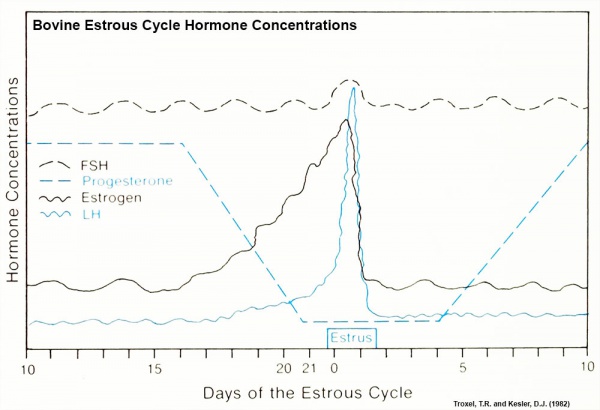
Bovine Estrous Cycle
Specific hormone concentrations are not shown in the above graph, only the relative hormone levels at different times during the cycle.
- Links: Estrous Cycle
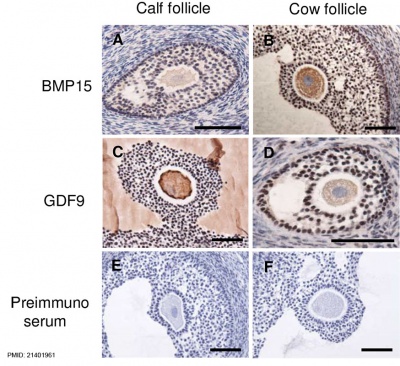
Oocyte Development
Bovine ovarian follicle BMP15 and GDF9 expression[12]
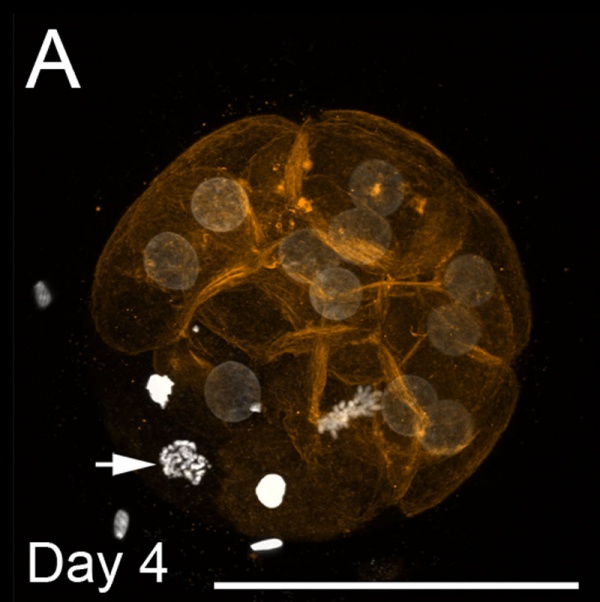
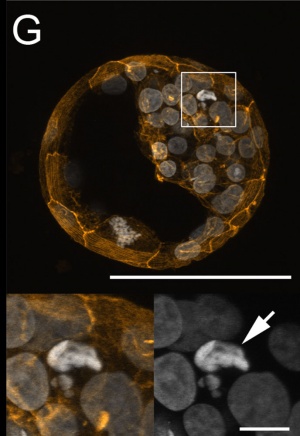
Morula and Blastocyst

Bovine Morula (day 4)[13]
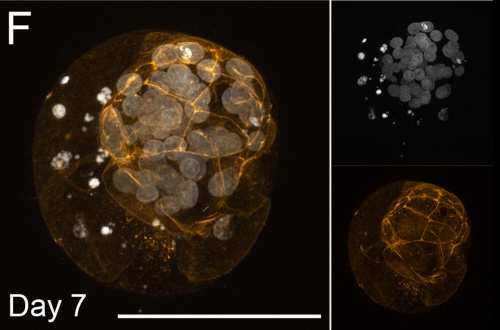
Bovine Blastocyst (day 7)[13]
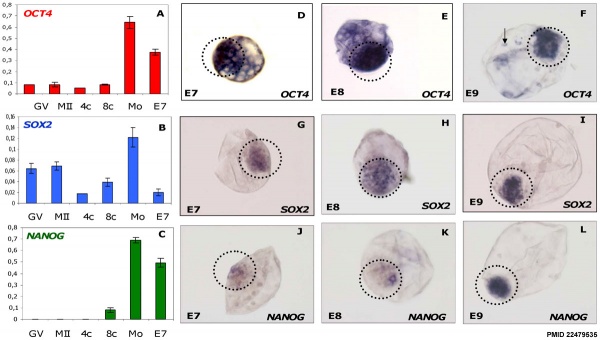
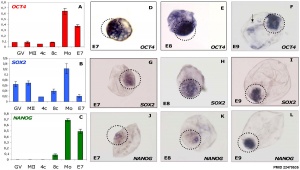
Bovine stem cell marker expression[2]
- Links: Image - Morula and Blastocyst | Morula A | Blastocyst F | Blastocyst G | Bovine Development | Morula | Blastocyst
Placenta
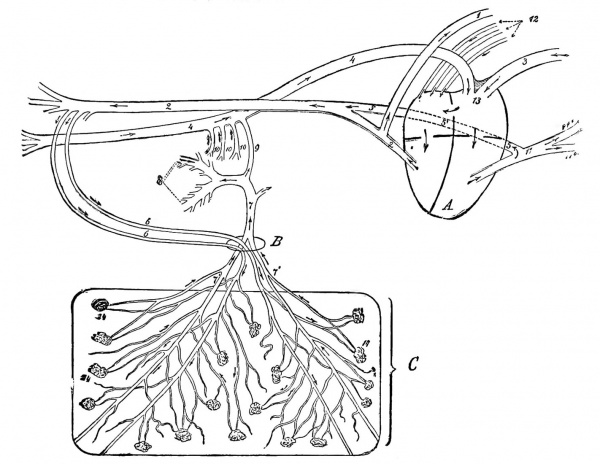
|
Fetal Circulation of a Calf
Placentation is epitheliochorial, where the maternal epithelium of the uterus comes in contact with the chorion, considered as primitive. The arrows indicate the direction in which the blood flows. A, Heart; B, umbilical opening; C, portion of the chorion. 1, Anterior aorta; 2, posterior aorta; 3 anterior vena cava; 4, posterior vena cava; 5, duct of Botalli; part of Botalli's duct posterior to the heart (sketched somewhat too long, but was necessary in order to demonstrate it) ; 6, umbilical arteries; 7, umbilical vein; 7', some of its branches; 8, portal vein; 9, ductus venosus; 10, portal veins: 11, pulmonary artery; 11', some of its branches; 12, pulmonary veins; 13, tuberculum Loweri; 14, chorion papillae. Figure: DeBruin Bovine Obstetrics (1910)
|
Genital Development
Testis
The male bovine (bull) first development of the testis at the genital ridge is triggered by SRY expression following the timeline shown below.[14]
- Day 32 - (CRL 12) Genital ridges first appeared
- Day 37 - (CRL 18) SRY expression begins
- Day 39 - (CRL 20) SRY expression peaks
- Day 42 - (CRL 27) Testis cords distinguishable
- Links: Testis Development
Ovary
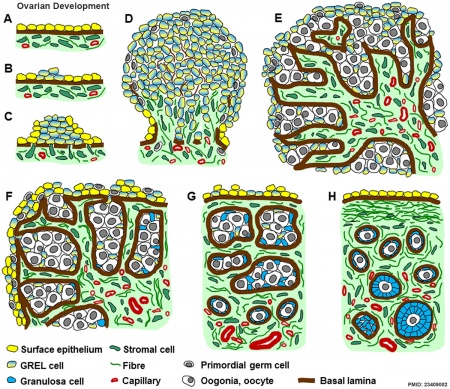
|
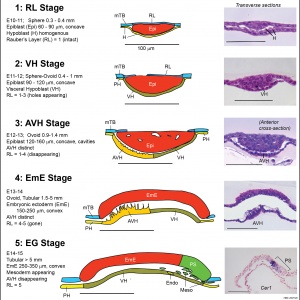
Ovarian Development Model[15]
|
References
- ↑ 1.0 1.1 van Leeuwen J, Berg DK & Pfeffer PL. (2015). Morphological and Gene Expression Changes in Cattle Embryos from Hatched Blastocyst to Early Gastrulation Stages after Transfer of In Vitro Produced Embryos. PLoS ONE , 10, e0129787. PMID: 26076128 DOI.
- ↑ 2.0 2.1 2.2 Khan DR, Dubé D, Gall L, Peynot N, Ruffini S, Laffont L, Le Bourhis D, Degrelle S, Jouneau A & Duranthon V. (2012). Expression of pluripotency master regulators during two key developmental transitions: EGA and early lineage specification in the bovine embryo. PLoS ONE , 7, e34110. PMID: 22479535 DOI.
- ↑ Krog CH, Agerholm JS & Nielsen SS. (2018). Fetal age assessment for Holstein cattle. PLoS ONE , 13, e0207682. PMID: 30452469 DOI.
- ↑ Negrón-Pérez VM, Rodrigues LT, Mingoti GZ & Hansen PJ. (2018). Role of ROCK Signaling in Formation of the Trophectoderm of the Bovine Preimplantation Embryo. Mol. Reprod. Dev. , , . PMID: 29542836 DOI.
- ↑ Gilchrist GC, Tscherner A, Nalpathamkalam T, Merico D & LaMarre J. (2016). MicroRNA Expression during Bovine Oocyte Maturation and Fertilization. Int J Mol Sci , 17, 396. PMID: 26999121 DOI.
- ↑ Oliveira CS, Saraiva NZ, de Lima MR, Oliveira LZ, Serapião RV, Garcia JM, Borges CA & Camargo LS. (2016). Cell death is involved in sexual dimorphism during preimplantation development. Mech. Dev. , 139, 42-50. PMID: 26752320 DOI.
- ↑ Salilew-Wondim D, Fournier E, Hoelker M, Saeed-Zidane M, Tholen E, Looft C, Neuhoff C, Besenfelder U, Havlicek V, Rings F, Gagné D, Sirard MA, Robert C, Shojaei Saadi HA, Gad A, Schellander K & Tesfaye D. (2015). Genome-Wide DNA Methylation Patterns of Bovine Blastocysts Developed In Vivo from Embryos Completed Different Stages of Development In Vitro. PLoS ONE , 10, e0140467. PMID: 26536655 DOI.
- ↑ Denicol AC, Leão BC, Dobbs KB, Mingoti GZ & Hansen PJ. (2015). Influence of Sex on Basal and Dickkopf-1 Regulated Gene Expression in the Bovine Morula. PLoS ONE , 10, e0133587. PMID: 26196299 DOI.
- ↑ Beindorff N, Nagai K, Shirasuna K, Herzog K, Hoeffmann K, Sasaki M, Bollwein H & Miyamoto A. (2010). Vascular changes in the corpus luteum during early pregnancy in the cow. J. Reprod. Dev. , 56, 263-70. PMID: 20103987
- ↑ Kues WA, Sudheer S, Herrmann D, Carnwath JW, Havlicek V, Besenfelder U, Lehrach H, Adjaye J & Niemann H. (2008). Genome-wide expression profiling reveals distinct clusters of transcriptional regulation during bovine preimplantation development in vivo. Proc. Natl. Acad. Sci. U.S.A. , 105, 19768-73. PMID: 19064908 DOI.
- ↑ Bruin, M. G. de. Bovine obstetrics (1910) translated by W. E. A. Wyman
- ↑ Hosoe M, Kaneyama K, Ushizawa K, Hayashi KG & Takahashi T. (2011). Quantitative analysis of bone morphogenetic protein 15 (BMP15) and growth differentiation factor 9 (GDF9) gene expression in calf and adult bovine ovaries. Reprod. Biol. Endocrinol. , 9, 33. PMID: 21401961 DOI.
- ↑ 13.0 13.1 13.2 Leidenfrost S, Boelhauve M, Reichenbach M, Güngör T, Reichenbach HD, Sinowatz F, Wolf E & Habermann FA. (2011). Cell arrest and cell death in mammalian preimplantation development: lessons from the bovine model. PLoS ONE , 6, e22121. PMID: 21811561 DOI.
- ↑ Ross DG, Bowles J, Hope M, Lehnert S & Koopman P. (2009). Profiles of gonadal gene expression in the developing bovine embryo. Sex Dev , 3, 273-83. PMID: 19844082 DOI.
- ↑ Hummitzsch K, Irving-Rodgers HF, Hatzirodos N, Bonner W, Sabatier L, Reinhardt DP, Sado Y, Ninomiya Y, Wilhelm D & Rodgers RJ. (2013). A new model of development of the mammalian ovary and follicles. PLoS ONE , 8, e55578. PMID: 23409002 DOI.
Reviews
Kropp J, Peñagaricano F, Salih SM & Khatib H. (2014). Invited review: Genetic contributions underlying the development of preimplantation bovine embryos. J. Dairy Sci. , 97, 1187-201. PMID: 24377798 DOI.
Aerts JM & Bols PE. (2010). Ovarian follicular dynamics: a review with emphasis on the bovine species. Part I: Folliculogenesis and pre-antral follicle development. Reprod. Domest. Anim. , 45, 171-9. PMID: 19210660 DOI.
Aerts JM & Bols PE. (2010). Ovarian follicular dynamics. A review with emphasis on the bovine species. Part II: Antral development, exogenous influence and future prospects. Reprod. Domest. Anim. , 45, 180-7. PMID: 19090819 DOI.
Gómez E, Caamaño JN, Rodríguez A, De Frutos C, Facal N & Díez C. (2006). Bovine early embryonic development and vitamin A. Reprod. Domest. Anim. , 41 Suppl 2, 63-71. PMID: 16984470 DOI.
Farin PW, Piedrahita JA & Farin CE. (2006). Errors in development of fetuses and placentas from in vitro-produced bovine embryos. Theriogenology , 65, 178-91. PMID: 16266745 DOI.
Farin CE, Farin PW & Piedrahita JA. (2004). Development of fetuses from in vitro-produced and cloned bovine embryos. J. Anim. Sci. , 82 E-Suppl, E53-62. PMID: 15471815 DOI.
Articles
O'Doherty AM, Magee DA, O'Shea LC, Forde N, Beltman ME, Mamo S & Fair T. (2015). DNA methylation dynamics at imprinted genes during bovine pre-implantation embryo development. BMC Dev. Biol. , 15, 13. PMID: 25881176 DOI.
Datta TK, Rajput SK, Wee G, Lee K, Folger JK & Smith GW. (2015). Requirement of the transcription factor USF1 in bovine oocyte and early embryonic development. Reproduction , 149, 203-12. PMID: 25385722 DOI.
Ibrahim S, Salilew-Wondim D, Rings F, Hoelker M, Neuhoff C, Tholen E, Looft C, Schellander K & Tesfaye D. (2015). Expression pattern of inflammatory response genes and their regulatory micrornas in bovine oviductal cells in response to lipopolysaccharide: implication for early embryonic development. PLoS ONE , 10, e0119388. PMID: 25764515 DOI.
Madeja ZE, Sosnowski J, Hryniewicz K, Warzych E, Pawlak P, Rozwadowska N, Plusa B & Lechniak D. (2013). Changes in sub-cellular localisation of trophoblast and inner cell mass specific transcription factors during bovine preimplantation development. BMC Dev. Biol. , 13, 32. PMID: 23941255 DOI.
Search Pubmed
Search Pubmed: bovine development
Terms
- Rauber layer - the thinned-out trophoblastic layer lying over the embryonic disk in developing carnivores and ungulates. Named after August A. Rauber (1841-1917) a German anatomist.
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Oklahoma State University Learning Reproduction in Farm Animals
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 19) Embryology Bovine Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Bovine_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G