Introduction
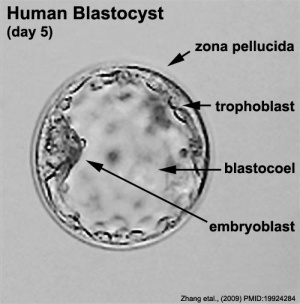
Human Blastocyst (Carnegie Stage 3)
The term "stem cells" is used so freely these days in many different forums that it is difficult sometimes understand without context what scientists, politicians, ethicists and commentators are discussing. In terms of human development, the embryonic stem cell with totipotential occurs at the blastocyst stage, mainly in the first and second week of development. After this period the inner cell mass, which forms the entire embryo, will differentiate into embryonic germ layers with restricted differentiation potential.
Stem cells as well as having the capacity to differentiate into any (totipotential) or multiple (pluripotential) cell types, have the unique capacity of self-renewal.
In vitro fertilization and growth of the blastocyst, allows isolation of these cells and their subsequent use in stem cell research. It is the collection, production and possible therapeutic applications of these stem cells which has recently attracted worldwide attention.
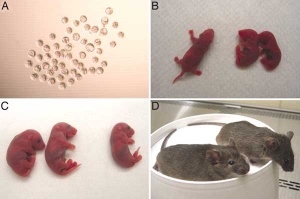
Mice cloned from adult keratinocytes
[1]A key step in the development of stem cell research has been the identification of cell surface markers (proteins) which identify these cells and their state of undifferentiation. A new area of research based upon stem cells has been teh development of in vitro culture organoids.
NIH Information
A useful guide (online PDF document) to stem cells was produced in a report by the National Institute of Health (NIH, USA, April 2009) Stem Cells: A Primer (PDF 1.89 MB) and more recently NIH has established a Stem Cell information page.
- Stem Cells: NIH 2009 Primer | File:NIH Regenerative Medicine 2006.pdf | 2001 Primer | NIH Stem Cell Basics | 2009 NIH Report | Regenerative Medicine 2006 | 2001 NIH Report
Some Recent Findings
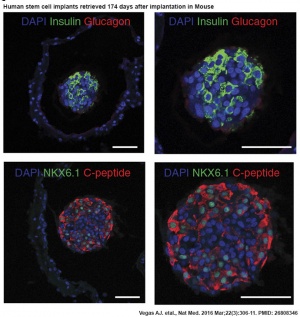
Human stem cell pancreas implants
[2]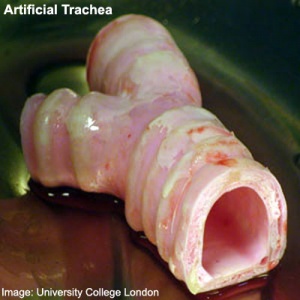
Stem cell artificial trachea and bronchi (Image
UCL)
- Deriving Human Naive Embryonic Stem Cell Lines from Donated Supernumerary Embryos Using Physical Distancing and Signal Inhibition[3] "Until recently, naïve pluripotent stem cell lines were not captured from human embryos because protocols were based upon those devised for murine embryonic stem cells. In contrast with early lineage segregation in mouse embryos, human hypoblast specification is not solely dependent upon FGF signaling; consequently, its maturation during embryo explant culture may provide inductive signals to drive differentiation of the epiblast. To overcome this potential risk, here we describe how cells of the immature inner cell mass of human embryos can be physically separated during derivation, achieved via "immunosurgery", to eliminate the trophectoderm, followed by disaggregation of the remaining inner cell mass cells. A modification of a culture regime developed for propagation of human pluripotent stem cells reset to the naïve state is used, which comprises serum-free medium supplemented with various inhibitors of signaling pathways, polarization, and differentiation. Colonies arising from the first plating of an inner cell mass may be pooled for ease of handling, or propagated separately to allow establishment of clonal human naïve embryonic stem cell lines."
- Adult tissue-derived neural crest-like stem cells[4] "neural crest (NC) cells are a multipotent stem cell population that gives rise to a diverse array of cell types in the body, including peripheral neurons, Schwann cells (SC), craniofacial cartilage and bone, smooth muscle cells, and melanocytes. NC formation and differentiation into specific lineages takes place in response to a set of highly regulated signaling and transcriptional events within the neural plate border. Pre-migratory NC cells initially are contained within the dorsal neural tube from which they subsequently emigrate, migrating to often distant sites in the periphery. Following their migration and differentiation, some NC-like cells persist in adult tissues in a nascent multipotent state, making them potential candidates for autologous cell therapy. This review discusses the gene regulatory network responsible for NC development and maintenance of multipotency. We summarize the genes and signaling pathways that have been implicated in the differentiation of a post-migratory NC into mature myelinating SC. We elaborate on the signals and transcription factors involved in the acquisition of immature SC fate, axonal sorting of unmyelinated neuronal axons, and finally the path toward mature myelinating SC, which envelope axons within myelin sheaths, facilitating electrical signal propagation. The gene regulatory events guiding development of SC in-vivo provides insights into means for differentiating NC-like cells from adult human tissues into functional SC, which have the potential to provide autologous cell sources for the treatment of demyelinating and neurodegenerative disorders."
- Generation of human oogonia from induced pluripotent stem cells in vitro[5] "Human pluripotent stem cells (hPSCs) have been induced into primordial germ cell-like cells (hPGCLCs); however, further differentiation to a mature germ cell has not been achieved. Here, we show that hPGCLCs differentiate progressively into oogonia-like cells during a long-term in vitro culture (~four months) in xenogeneic reconstituted ovaries with mouse embryonic ovarian somatic cells. The hPGCLC-derived oogonia display hallmarks of epigenetic reprogramming, i.e., genome-wide DNA demethylation, imprint erasure, and extinguishment of aberrant DNA methylation in hPSCs, and acquire an immediate precursory state for meiotic recombination. Furthermore, the inactive X chromosome shows a progressive demethylation and reactivation, albeit partially. These findings establish the germline competence of hPSCs and provide a critical step toward human in vitro gametogenesis." oocyte
|
| More recent papers
|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
- This search now requires a manual link as the original PubMed extension has been disabled.
- The displayed list of references do not reflect any editorial selection of material based on content or relevance.
- References also appear on this list based upon the date of the actual page viewing.
References listed on the rest of the content page and the associated discussion page (listed under the publication year sub-headings) do include some editorial selection based upon both relevance and availability.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References
Search term: Stem Cells
|
| Older papers
|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
- Establishment and characterization of human theca stem cells and their differentiation into theca progenitor cells[6] "In this study, we have characterized the human theca stem cells (hTSCs) and their differentiation into human theca progenitor cells (hTPCs). hTSCs were isolated from the theca layer of small antral follicles (3-5 mm in size)." ovary
- Distinct SoxB1 networks are required for naïve and primed pluripotency[7] "Deletion of Sox2 from mouse embryonic stem cells (ESCs) causes trophectodermal differentiation. While this can be prevented by enforced expression of the related SOXB1 proteins, SOX1 or SOX3, the roles of SOXB1 proteins in epiblast stem cell (EpiSC) pluripotency are unknown. Here, we show that Sox2 can be deleted from EpiSCs with impunity. This is due to a shift in the balance of SoxB1 expression in EpiSCs, which have decreased Sox2 and increased Sox3 compared to ESCs. Consistent with functional redundancy, Sox3 can also be deleted from EpiSCs without eliminating self-renewal. However, deletion of both Sox2 and Sox3 prevents self-renewal. The overall SOXB1 levels in ESCs affect differentiation choices: neural differentiation of Sox2 heterozygous ESCs is compromised, while increased SOXB1 levels divert the ESC to EpiSC transition towards neural differentiation. Therefore, optimal SOXB1 levels are critical for each pluripotent state and for cell fate decisions during exit from naïve pluripotency." Sox
- Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice[2] "The transplantation of glucose-responsive, insulin-producing cells offers the potential for restoring glycemic control in individuals with diabetes. Pancreas transplantation and the infusion of cadaveric islets are currently implemented clinically, but these approaches are limited by the adverse effects of immunosuppressive therapy over the lifetime of the recipient and the limited supply of donor tissue. The latter concern may be addressed by recently described glucose-responsive mature beta cells that are derived from human embryonic stem cells (referred to as SC-β cells), which may represent an unlimited source of human cells for pancreas replacement therapy. ...human SC-β cells were encapsulated with alginate derivatives capable of mitigating foreign-body responses in vivo and implanted into the intraperitoneal space of C57BL/6J mice treated with streptozotocin, which is an animal model for chemically induced type 1 diabetes. These implants induced glycemic correction without any immunosuppression until their removal at 174 d after implantation. Human C-peptide concentrations and in vivo glucose responsiveness demonstrated therapeutically relevant glycemic control. Implants retrieved after 174 d contained viable insulin-producing cells."
- Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1[8] "Haematopoietic stem cells (HSCs) are self-renewing stem cells capable of replenishing all blood lineages. In all vertebrate embryos that have been studied, definitive HSCs are generated initially within the dorsal aorta (DA) of the embryonic vasculature by a series of poorly understood inductive events. Previous studies have identified that signalling relayed from adjacent somites coordinates HSC induction, but the nature of this signal has remained elusive. Here we reveal that somite specification of HSCs occurs via the deployment of a specific endothelial precursor population, which arises within a sub-compartment of the zebrafish somite that we have defined as the endotome. Endothelial cells of the endotome are specified within the nascent somite by the activity of the homeobox gene meox1. Specified endotomal cells consequently migrate and colonize the DA, where they induce HSC formation through the deployment of chemokine signalling activated in these cells during endotome formation." blood
- Generation of organized germ layers from a single mouse embryonic stem cell[9] "Mammalian inner cell mass cells undergo lineage-specific differentiation into germ layers of endoderm, mesoderm and ectoderm during gastrulation. It has been a long-standing challenge in developmental biology to replicate these organized germ layer patterns in culture. Here we present a method of generating organized germ layers from a single mouse embryonic stem cell cultured in a soft fibrin matrix." gastrulation
- Derivation of naive human embryonic stem cells[10] "We show that human naïve cells meet mouse criteria for the naïve state by growth characteristics, antibody labeling profile, gene expression, X-inactivation profile, mitochondrial morphology, microRNA profile and development in the context of teratomas. hESCs can exist in a naïve state without the need for transgenes. Direct derivation is an elusive, but attainable, process, leading to cells at the earliest stage of in vitro pluripotency described for humans. Reverse toggling of primed cells to naïve is efficient and reproducible."
- Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer [11] 1. Cytoplasm of human oocytes reprograms transplanted somatic cell nuclei to pluripotency. 2. NT-ESCs can be efficiently derived from high-quality human oocytes 3. Human NT-ESCs are similar to ESCs derived from fertilized embryos. Nature comment - Human stem cells created by cloning
- The Nobel Prize in Physiology or Medicine 2012 was awarded jointly to Sir John B. Gurdon and Shinya Yamanaka "for the discovery that mature cells can be reprogrammed to become pluripotent" Shinya Yamanaka Yamanaka Factors are a set of 4 transcription factors when introduced into cells induces stem cell formation. John Gurdon used nuclear transplantation and cloning to show that the nucleus of a differentiated somatic cell retains the totipotency necessary to form a whole organism. Induced Stem Cells
- Nature Cell Biology Focus on stem cells "This issue presents a series of specially commissioned articles that highlight exciting facets of stem cell research, including recent insights into the nature of pluripotency and how studying stem cells can increase our understanding of normal ageing and disease." Editorial
- First Successful Transplantation of a Synthetic Tissue Engineered Windpipe Karolinska Institute | University College London | BBC News "An international team designed and built the nanocomposite tracheal scaffold and produced a specifically designed bioreactor used to seed the scaffold with the patient´s own stem cells. The cells were grown on the scaffold inside the bioreactor for two days before transplantation to the patient. Because the cells used to regenerate the trachea were the patient's own, there has been no rejection of the transplant and the patient is not taking immunosuppressive drugs."
- Culture of human pluripotent stem cells using completely defined conditions on a recombinant E-cadherin substratum[12] "huES and human induced pluripotent stem (hiPS) cells were grown on plates coated with a fusion protein consisting of E-cadherin and the IgG Fc domain using mTeSR1 medium. Cells grown under these conditions maintained similar morphology and growth rate to those grown on Matrigel and retained all pluripotent stem cell features, including an ability to differentiate into multiple cell lineages in teratoma assays."
- Epigenetic memory in induced pluripotent stem cells.[13] "Our data indicate that nuclear transfer is more effective at establishing the ground state of pluripotency than factor-based reprogramming, which can leave an epigenetic memory of the tissue of origin that may influence efforts at directed differentiation for applications in disease modelling or treatment."
|
Embryonic Stem Cell
Mesenchymal Stem Cells
Recently the human GA 14 to 16 weeks fetal heart have been used as a source of mesenchymal stem cells that appear similar to human bone marrow mesenchymal stem cells (expressing CD73, CD90, CD105 and lacking expression of CD31, CD34, CD45, HLA-DR).[14]
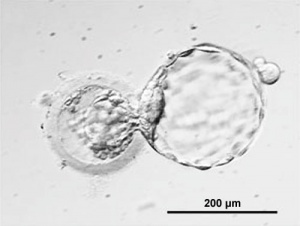
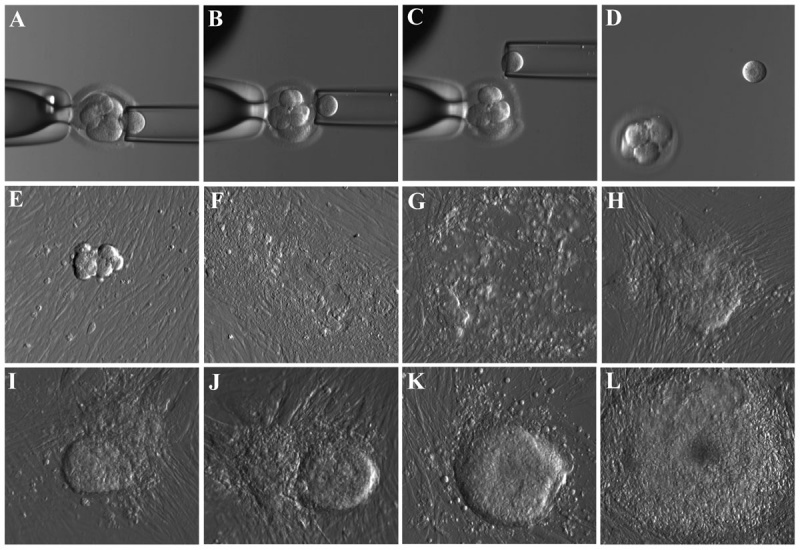
Human blastocyst derived stem cells[15]
(A–D) - stepwise procedure of embryo biopsy using inverted microscope-attached micro manipulator.
(E–L) - appearance of initial outgrowth and hESC colony during the derivation procedure.
Cord Blood Stem Cell
Placental cord blood is a rich souce of haematopoietic stem cells for transplantation. Cord blood can collected at birth, with no impact on the mother or neonate, and stured in cord blood banks for later use. BBC (UK) A brief article on Cord Blood stem cells and their therapeutic potential.
- Links: Stem Cells - Placental Cord Blood
Spermatogonial Stem Cell (SSC)
In the male testes are a population of spermatogonia cells that differentiate and meiotically divide to form spermatozoa cells (male germ cells).
- Production of knockout mice by random or targeted mutagenesis in spermatogonial stem cells.[16]
- Spermatogonial stem cells: questions, models and perspectives.[17]
- [Spermatogonial stem cells: characteristics and experimental possibilities.[18]
- Genetic and epigenetic properties of mouse male germline stem cells during long-term culture.[19]
- Expansion of murine spermatogonial stem cells through serial transplantation.[20]
Adult Stem Cell
[[File:Epidermis-stem cell models.jpg|thumb|Epidermis - stem cell models[21]
Adult stem cells, with pluropotentiality, are found in several body systems: intestinal epithelium, epidermis, testis and bone marrow.
- Generation of pluripotent stem cells from adult human testis[22] "Human primordial germ cells and mouse neonatal and adult germline stem cells are pluripotent and show similar properties to embryonic stem cells. Here we report the successful establishment of human adult germline stem cells derived from spermatogonial cells of adult human testis."
- Links: Stem Cells - Adult
Inducible Stem Cells
Inducible pluripotent stem cells (iPS) require a minimum of key defined transcription factors (Oct3/4, Sox2, Klf4, c-Myc, Nanog and Lin28) are required to be introduced into a cell to "induce" that cell to revert to a stem cell phenotype.
- Induction of pluripotent stem cells from adult human fibroblasts by defined factors.[23]
- Generation of induced pluripotent stem cells by reprogramming mouse embryonic fibroblasts with a four transcription factor, doxycycline inducible lentiviral transduction system.[24]
- Links: Stem Cells - Induced
Nuclear Transfer
This technique involves removing the nucleus from an early stage embryo and replacing with the nucleus from another cell. If the replacement nucleus is from a somatic cell, not a gamete, the technique is also described as somatic cell nuclear transfer (SCNT). The most famous of which was the sheep "Dolly". More recently nuclei have been sourced from a number of different tissues, including those from long-term frozen animals.[25] See also a review of this technique.[26]
- Links: Somatic Cell Nuclear Transfer | Stem Cells - SCNT
Stem Cell Regulation
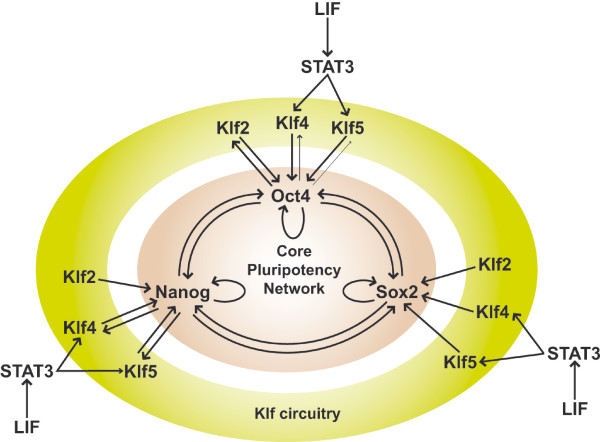
Embryonic stem cell signaling regulation (mouse)[27]
Stem Cell Markers
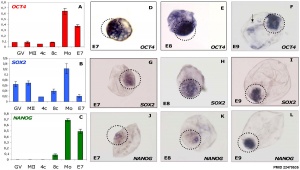
Bovine stem cell marker expression
[28]In order to carry out research on stem cells, it is important to be able to identify them. A number of different research groups in the late 90's generated several antibodies which specifically identified undifferentiated, differentiating or differentiated stem cells from a number of different sources and species. Note that the nomenclature in some cases is based upon the antibody used to identify the cell surface marker.
- Stage-Specific Embryonic Antigen-1 (SSEA-1) cell surface embryonic antigen which has a role in cell adhesion, migration and differentiation and is often differentially expressed during development. Can be identified by Davor Solter (monoclonal antibody MC-480) (SSEA-1).
- Stage-Specific Embryonic Antigen-4 (SSEA-4) cell surface embryonic antigen of human teratocarcinoma stem cells (EC), human embryonic germ cells (EG) and human embryonic stem cells (ES) which is down-regulated following differentiation of human EC cells. Antigen not expressed on undifferentiated murine EC, ES and EG cells but upregulated on differentiation of murine EC and ES cells. Can be identified by Davor Solter (monoclonal antibody MC-813-70) (SSEA-4)
- Tumor Rejection Antigen (TRA-1-60) Sialylated Keratan Sulfate Proteoglycan expressed on the surface of human teratocarcinoma stem cells (EC), human embryonic germ cells (EG) and human embryonic stem cells (ES).
- Tumor Rejection Antigen (TRA-1-81) antigen expressed on the surface of human teratocarcinoma stem cells (EC), human embryonic germ cells (EG) and human embryonic stem cells (ES). Both TRA antibodies identify a major polypeptide (Mr 240 kDa) and a minor polypeptide (Mr 415 kDa).
- Oct-4 (Pou5f1) gene has an essential role in control of developmental pluripotency (Oct4 knockout embryo blastocysts die at the time of implantation). Oct4 also has a role in maintaining viability of mammalian germline.
- Stem Cell Antigen 1 (Sca-1) member of the Ly-6 family of GPI-linked surface proteins (Mr 18 kDa) and a major phenotypic marker for mouse hematopoietic progenitor/stem cell subset.
- CD133, AC133, prominin 5 transmembrane glycoprotein (865 aa) expressed on stem cells with hematopoietic and nonhematopoietic differentiation potential.
- Alpha 6 integrin
Data based on information from Appendix E.II. NIH Report "Stem Cells: Scientific Progress and Future Research Directions", Chemicon International- Stem cell marker antibodies OMIM and other sources.
Human Embryonic Stem Cell Markers
A recent paper identified the expression pattern of a new human embryonic stem cell line (hESC).[29]
- alkaline phosphatase
- human telomerase reverse transcriptase
- SSEA-3, SSEA-4
- TRA-1-60, TRA-1-81
- OCT-4, Nanog
- Rex-1, Sox-2, UTF-1, Connexins 43 and 45
- TERF-1 and TERF-2
- Glut-1, BCRP-1/ABCG-2, GDF3, LIN28, FGF4, Thy-1
- Cripto1/TDGF1, AC133
- SMAD1/2/3/5
Opinion on Stem Cell Use
Results from a recent Australian survey into couples' views on the use of supernumerary embryos:[30]
- 40% (123/311) returned completed questionnaires.
- 42% most common decision was donation to research (altruistic motives and desire not to waste embryos were determinants of embryo donation).
Determinants of disposal were not wanting a full sibling to existing children and opposition of embryo research.
- 45% found deciding distressing.
- 69% approved of embryo donation to stem-cell research.
Stem Cell Fake Result
Hwang Woo-suk (Korean pioneer of stem cell research) Resigns A Seoul National University investigation of the original data in Science paper Jun (2005;308: 1777-83) "Eleven human embryonic stem cells (hESC) lines were established by nuclear transfer (SCNT; NT) of skin cells from patients with disease or injury into donated oocytes." announced 29 Dec 2005 that he had faked the results.
The journal Science retracted the original paper, the original reference with link to the erratum.[31]
Science News 06 Jan | Special Online Collection: Hwang et al. and Stem Cell Issues
Cancer
There is a hypothesis that several cancers may arise from somatic stem or progenitor cells that exist in different tissues. These cancer stem cells are called "side population" (SP) cells and have been identified in: leukemia, breast cancer and several human cancer cell lines (central nervous system, gastrointestinal tumors, retinoblastoma). There is still a "chicken and egg" problem to be resolved, in that the cancer cells may have dedifferentiated to a stem cell-like population.
A recent paper has also identified SP cells in ovarian cancer which have properties similar to stem cells.[32]
Cell Types
The tables below are provided only as a guide.
Adult Human Cell Types
| Adult Human Cell Types
|
| Adult Human Cell Types
|
Integumentary system
- Keratinizing epithelial cells
- Epidermal keratinocyte (differentiating epidermal cell)
- Epidermal basal cell (stem cell)
- Keratinocyte of fingernails and toenails
- Nail bed basal cell (stem cell)
- Medullary hair shaft cell
- Cortical hair shaft cell
- Cuticular hair shaft cell
- Cuticular hair root sheath cell
- Hair root sheath cell of Huxley's layer
- Hair root sheath cell of Henle's layer
- External hair root sheath cell
- Hair matrix cell (stem cell)
|
Wet stratified barrier epithelial cells
- Surface epithelial cell of stratified squamous epithelium of cornea, tongue, oral cavity, esophagus, anal canal, distal urethra and vagina
- Basal cell (stem cell) of epithelia of cornea, tongue, oral cavity, esophagus, anal canal, distal urethra and vagina Urinary epithelium cell (lining urinary bladder and urinary ducts)
|
Exocrine secretory epithelial cells
- Salivary gland mucous cell (polysaccharide-rich secretion)
- Salivary gland serous cell (glycoprotein enzyme-rich secretion)
- Von Ebner's gland cell in tongue (washes taste buds)
- Mammary gland cell (milk secretion)
- Lacrimal gland cell (tear secretion)
- Ceruminous gland cell in ear (wax secretion)
- Eccrine sweat gland dark cell (glycoprotein secretion)
- Eccrine sweat gland clear cell (small molecule secretion)
- Apocrine sweat gland cell (odoriferous secretion, sex-hormone sensitive)
- Gland of Moll cell in eyelid (specialized sweat gland)
- Sebaceous gland cell (lipid-rich sebum secretion)
- Bowman's gland cell in nose (washes olfactory epithelium)
- Brunner's gland cell in duodenum (enzymes and alkaline mucus)
- Seminal vesicle cell (secretes seminal fluid components, including fructose for swimming sperm)
- Prostate gland cell (secretes seminal fluid components)
- Bulbourethral gland cell (mucus secretion)
- Bartholin's gland cell (vaginal lubricant secretion)
- Gland of Littre cell (mucus secretion)
- Uterus endometrium cell (carbohydrate secretion)
- Isolated goblet cell of respiratory and digestive tracts (mucus secretion) Stomach lining mucous cell (mucus secretion)
- Gastric gland zymogenic cell (pepsinogen secretion)
- Gastric gland oxyntic cell (hydrochloric acid secretion)
- Pancreatic acinar cell (bicarbonate and digestive enzyme secretion)
- Paneth cell of small intestine (lysozyme secretion)
- Type II pneumocyte of lung (surfactant secretion) Clara cell of lung
|
Hormone secreting cells
- Anterior pituitary cells Somatotropes
- Lactotropes
- Thyrotropes Gonadotropes Corticotropes
- Intermediate pituitary cell, secreting melanocyte-stimulating hormone Magnocellular neurosecretory cells
- secreting oxytocin
- secreting vasopressin Gut and respiratory tract cells
- secreting serotonin secreting endorphin secreting somatostatin secreting gastrin secreting secretin secreting cholecystokinin secreting insulin secreting glucagon secreting bombesin
- Thyroid gland cells thyroid epithelial cell
- parafollicular cell Parathyroid gland cells
- Parathyroid chief cell
- Oxyphil cell Adrenal gland cells
- chromaffin cells
- secreting steroid hormones (mineralcorticoids and gluco corticoids) Leydig cell of testes secreting testosterone Theca interna cell of ovarian follicle secreting oestrogen
- Corpus luteum cell of ruptured ovarian follicle secreting progesterone
- Granulosa lutein cells
- Theca lutein cells
- Juxtaglomerular cell (renin secretion)
- Macula densa cell of kidney
- Peripolar cell of kidney
- Mesangial cell of kidney
|
Metabolism and storage cells
- Hepatocyte (liver cell) White fat cell Brown fat cell Liver lipocyte
|
| Barrier function cells (Lung, Gut, Exocrine Glands and Urogenital Tract)
Kidney
- Kidney glomerulus parietal cell
- Kidney glomerulus podocyte
- Kidney proximal tubule brush border cell
- Loop of Henle thin segment cell
- Kidney distal tubule cell
- Kidney collecting duct cell
Other
- Type I pneumocyte (lining air space of lung)
- Pancreatic duct cell (centroacinar cell)
- Nonstriated duct cell (of sweat gland, salivary gland, mammary gland, etc.)
- principal cell
- Intercalated cell Duct cell (of seminal vesicle, prostate gland, etc.)
- Intestinal brush border cell (with microvilli)
- Exocrine gland striated duct cell
- Gall bladder epithelial cell
- Ductulus efferens nonciliated cell
- Epididymal principal cell
- Epididymal basal cell
Epithelial cells lining closed internal body cavities
- Blood vessel and lymphatic vascular endothelial fenestrated cell
- Blood vessel and lymphatic vascular endothelial continuous cell
- Blood vessel and lymphatic vascular endothelial splenic cell
- Synovial cell (lining joint cavities, hyaluronic acid secretion)
Serosal cell (lining peritoneal, pleural, and pericardial cavities)
Squamous cell (lining perilymphatic space of ear)
Squamous cell (lining endolymphatic space of ear)
Columnar cell of endolymphatic sac with microvilli (lining endolymphatic space of ear)
Columnar cell of endolymphatic sac without microvilli (lining endolymphatic space of ear)
Dark cell (lining endolymphatic space of ear)
Vestibular membrane cell (lining endolymphatic space of ear)
Stria vascularis basal cell (lining endolymphatic space of ear)
Stria vascularis marginal cell (lining endolymphatic space of ear)
Cell of Claudius (lining endolymphatic space of ear)
Cell of Boettcher (lining endolymphatic space of ear)
Choroid plexus cell (cerebrospinal fluid secretion)
Pia-arachnoid squamous cell
Pigmented ciliary epithelium cell of eye
Nonpigmented ciliary epithelium cell of eye
Corneal endothelial cell
|
Ciliated cells with propulsive function
- Respiratory tract ciliated cell
- Oviduct ciliated cell (in female)
- Uterine endometrial ciliated cell (in female)
- Rete testis ciliated cell (in male)
- Ductulus efferens ciliated cell (in male)
- Ciliated ependymal cell of central nervous system (lining brain cavities)
|
Extracellular matrix secretion cells
- Ameloblast epithelial cell (tooth enamel secretion)
- Planum semilunatum epithelial cell of vestibular apparatus of ear (proteoglycan secretion)
- Organ of Corti interdental epithelial cell (secreting tectorial membrane covering hair cells)
- Loose connective tissue fibroblasts
- Corneal fibroblasts
- Tendon fibroblasts
- Bone marrow reticular tissue fibroblasts
- Other nonepithelial fibroblasts
- Pericyte
- Nucleus pulposus cell of intervertebral disc
- Cementoblast/cementocyte (tooth root bonelike cementum secretion)
- Odontoblast/odontocyte (tooth dentin secretion)
- Hyaline cartilage chondrocyte
- Fibrocartilage chondrocyte
- Elastic cartilage chondrocyte Osteoblast/osteocyte
- Osteoprogenitor cell (stem cell of osteoblasts)
- Hyalocyte of vitreous body of eye
- Stellate cell of perilymphatic space of ear
|
Contractile cells
- Skeletal muscle cells
- Red skeletal muscle cell (slow)
- White skeletal muscle cell (fast)
- Intermediate skeletal muscle cell nuclear bag cell of muscle spindle nuclear chain cell of muscle spindle
- Satellite cell (stem cell)
- Heart muscle cells
- Ordinary heart muscle cell
- Nodal heart muscle cell
- Purkinje fiber cell
- Smooth muscle cell (various types)
- Myoepithelial cell of iris
- Myoepithelial cell of exocrine glands
|
Blood and immune system cells
- Erythrocyte (red blood cell)
- Megakaryocyte (platelet precursor)
- Monocyte Connective tissue macrophage (various types)
- Epidermal Langerhans cell
- Osteoclast (in bone)
- Dendritic cell (in lymphoid tissues)
- Microglial cell (in central nervous system)
- Neutrophil granulocyte
- Eosinophil granulocyte
- Basophil granulocyte
- Mast cell Helper
- T cell Suppressor
- T cell Cytotoxic
- T cell Natural
- Killer T cell
- B cell
- Natural killer cell
- Reticulocyte
- Stem cells and committed progenitors for the blood and immune system (various types)
|
Nervous system Sensory transducer cells
- Auditory inner hair cell of organ of Corti
- Auditory outer hair cell of organ of Corti
- Basal cell of olfactory epithelium (stem cell for olfactory neurons)
- Cold-sensitive primary sensory neurons
- Heat-sensitive primary sensory neurons
- Merkel cell of epidermis (touch sensor)
- Olfactory receptor neuron
- Pain-sensitive primary sensory neurons (various types)
- Photoreceptor cells of retina in eye:
- Photoreceptor rod cells
- Photoreceptor blue-sensitive cone cell of eye
- Photoreceptor green-sensitive cone cell of eye
- Photoreceptor red-sensitive cone cell of eye
- Proprioceptive primary sensory neurons (various types)
- Touch-sensitive primary sensory neurons (various types)
- Type I carotid body cell (blood pH sensor)
- Type II carotid body cell (blood pH sensor)
- Type I hair cell of vestibular apparatus of ear (acceleration and gravity)
- Type II hair cell of vestibular apparatus of ear (acceleration and gravity)
- Type I taste bud cell
|
Autonomic neuron cells
- Cholinergic neural cell (various types)
- Adrenergic neural cell (various types)
- Peptidergic neural cell (various types)
|
Sense organ and peripheral neuron supporting cells
- Inner pillar cell of organ of Corti
- Outer pillar cell of organ of Corti
- Inner phalangeal cell of organ of Corti
- Outer phalangeal cell of organ of Corti
- Border cell of organ of Corti
- Hensen cell of organ of Corti
- Vestibular apparatus supporting cell
- Type I taste bud supporting cell
- Olfactory epithelium supporting cell Schwann cell
- Satellite cell (encapsulating peripheral nerve cell bodies)
- Enteric glial cell
|
Central nervous system neurons and glial cells
- Neuron cells (large variety of types, still poorly classified)
- Spindle neuron
- Astrocyte (various types)
- Oligodendrocyte
|
Lens cells
- Anterior lens epithelial cell
- Crystallin-containing lens fiber cell
|
Pigment cells
- Melanocyte Retinal pigmented epithelial cell
|
Germ cells
- Oogonium/Oocyte
- Spermatid Spermatocyte Spermatogonium cell (stem cell for spermatocyte) Spermatozoon
|
Nurse cells
- Ovarian follicle cell Sertoli cell (in testis) Thymus epithelial cell
|
Interstitial cells
- Interstitial kidney cells
|
| Table based on MBoC - "There are about 210 known distinct human cell types.
|
- Links: Adult Human Cell Types | Ectoderm Cell Types | Mesoderm Cell Types | Endoderm Cell Types | | ectoderm | mesoderm | endoderm | stem cells
|
|
Ectoderm Cell Types
| Ectoderm Derived Cell Types
|
| Ectoderm Derived Cell Types
|
| integumentary
|
Keratinizing epithelial cells
- Epidermal keratinocyte (differentiating Epidermis (skin)|epidermal cell)
- Epidermal basal cell (Template:Stem cell)
- Keratinocyte of fingernails and toenails
- Nail bed basal cell (stem cell)
- Medullary hair shaft cell
- Cortical hair shaft cell
- Cuticular hair shaft cell
- Cuticular hair root sheath cell
- hair root sheath cell of Huxley's layer
- hair root sheath cell of Henle's layer
- External hair root sheath cell
- hair matrix cell (Template:Stem cell)
Wet stratified barrier epithelial cells
- Surface epithelial cell of stratified squamous epithelium of cornea, tongue, human oral cavity, esophagus, anal canal, distal urethra and vagina
- basal cell (stem cell) of epithelia of cornea, tongue, oral cavity, esophagus, anal canal, distal urethra and vagina
- Urinary epithelium cell (lining urinary bladder and urinary ducts)
|
| sensory
|
Sensory transducer cells
- Hair cell - Auditory inner hair cell of organ of Corti
- Hair cell - Auditory outer hair cell of organ of Corti
- smell olfactory epithelium basal cell (stem cell for olfactory neurons)
- Cold-sensitive primary sensory neurons
- Heat-sensitive primary sensory neurons
- Merkel cell of epidermis (touch sensor)
- Olfactory receptor neuron
- Pain-sensitive primary sensory neurons (various types)
- retina photoreceptors
- Photoreceptor rod cells
- Photoreceptor blue-sensitive cone cell of eye
- Photoreceptor green-sensitive cone cell of eye
- Photoreceptor red-sensitive cone cell of eye
- Proprioceptive primary sensory neurons (various types)
- touch-sensitive primary sensory neurons (various types)
- Type I carotid body cell (blood pH sensor)
- Type II carotid body cell (blood pH sensor)
- Type I hair cell of vestibular system of ear (acceleration and gravity)
- Type II hair cell of vestibular system of ear (acceleration and gravity)
- Type I taste bud|taste bud cell
|
Peripheral Nervous System
- Cholinergic neural cell (various types)
- Adrenergic neural cell (various types)
- Peptidergic neural cell (various types)
|
sensory and peripheral neuron supporting cells
- Inner pillar cell of organ of Corti
- Outer pillar cell of organ of Corti
- Inner phalangeal cell of organ of Corti
- Outer phalangeal cell of organ of Corti
- Border cell of organ of Corti
- Hensen cell of organ of Corti
- Vestibular apparatus supporting cell
- Taste bud supporting cell
- Olfactory epithelium supporting cell
- Schwann cell
- Satellite glial cell (encapsulating peripheral nerve cell bodies)
- Enteric glial cell
|
neural
- Neuron cells (large variety of types, still poorly classified)
- Interneurons
- Basket cells
- Stellate cells
- Golgi cells
- Granule cells
- Lugaro cells
- Unipolar brush cells
- Martinotti cells
- Chandelier cells
- Medium spiny neurons
- Cajal–Retzius cells
- Double-bouquet cells
- Neurogliaform cells
- Spinal interneuron
- Principal cells
- Spindle neuron
- Pyramidal cells
- Place cells
- Grid cells
- Speed cells
- Head direction cells
- Betz cells
- Stellate cells
- astroglia (various types)
- oligodendroglia
- Ependymal cells
|
lens cells
- Anterior lens epithelial cell
- Crystallin-containing lens fiber cell
|
- Links: ectoderm | Adult Human Cell Types | Ectoderm Cell Types | Mesoderm Cell Types | Endoderm Cell Types | stem cells
|
|
Mesoderm Cell Types
| Mesoderm Derived Cell Types
|
| mesoderm Derived Cell Types
|
Metabolism and storage cells
- Adipocytes
- Liver lipocyte
|
| Barrier function cells
(respiratory, gastrointestinal tract, urogenital tract)
|
renal
- Kidney parietal cell
- Kidney glomerulus podocyte
- Kidney proximal tubule brush border cell
- Loop of Henle thin segment cell
- Kidney distal tubule cell
- Kidney collecting duct cell
- Principal cells
- Intercalated cells
|
Other
- Type I pneumocyte (lining air space of lung cell)
- Pancreatic duct cell (centroacinar cell)
- Non-striated duct cell (of sweat gland, salivary gland, mammary gland, etc.)
- Principal cell
- Intercalated cell
- Duct cell (of seminal vesicle, prostate gland, etc.)
- Intestinal brush border cell (with microvilli)
- Exocrine gland striated duct cell
- Gall bladder epithelial cell
- Ductulus efferens non-ciliated cell
- Epididymal principal cell
- Epididymal basal cell
- Endothelial cells
|
Extracellular matrix cells
- Ameloblast epithelial cell (tooth enamel secretion)
- Planum semilunatum epithelial cell of vestibular system of ear (proteoglycan secretion)
- Organ of Corti interdental epithelial cell (secreting tectorial membrane covering hair cells)
- Loose connective tissue fibroblasts
- Corneal fibroblasts (corneal keratocytes)
- Tendon fibroblasts
- Bone marrow reticular tissue fibroblasts
- Other nonepithelial fibroblasts
- Pericyte
- Nucleus pulposus cell of intervertebral disc
- Cementoblast/cementocyte (tooth root bone-like ewan cell secretion)
- Odontoblast/odontocyte (tooth dentin secretion)
- Hyaline cartilage chondrocyte
- Fibrocartilage chondrocyte
- Elastic cartilage chondrocyte
- Osteoblast/osteocyte
- Osteoblast (osteoprogenitor cell, stem cell of osteoblasts)
- Hyalocyte of vitreous body of eye
- Stellate cell of perilymphatic space of ear
- Hepatic stellate cell (Ito cell)
- Pancreatic stelle cell
|
Contractile cells
- Skeletal muscle cell
- Red skeletal muscle cell (slow)
- White skeletal muscle cell (fast)
- Intermediate skeletal muscle cell
- Nuclear bag cell of muscle spindle
- Nuclear chain cell of muscle spindle
- Satellite cell (stem cell)
- Heart muscle cells
- Ordinary Myocardiocyte
- Nodal heart muscle cell
- Purkinje fiber cell
- Smooth muscle cell (various types)
- Myoepithelial cell of Iris
- Myoepithelial cell of exocrine glands
|
Blood and immune system cells
- Erythrocyte (red blood cell)
- Megakaryocyte (platelet precursor)
- Monocyte (white blood cell )
- Connective tissue macrophage (various types)
- Epidermal Langerhans cell
- Osteoclast (in bone)
- Dendritic cell (in lymphoid tissues)
- Microglial cell (in central nervous system)
- Neutrophil granulocyte
- Eosinophil granulocyte
- Basophil granulocyte
- Hybridoma cell (man-made)
- Mast cell
- Helper T cell
- Suppressor T cell
- Cytotoxic T cell
- Natural killer T cell
- B cell
- Natural killer cell
- Reticulocyte
- Stem cells and Progenitor cells (committed progenitors for the blood and immune system, various types)
|
| Germ cells
|
Nurse cell
- Ovarian follicle cell
- Sertoli cell (in testis)
- Thymus epithelial cell
|
Interstitial cells
- Interstitial kidney cells
|
- Links: mesoderm | Adult Human Cell Types | Ectoderm Cell Types | Mesoderm Cell Types | Endoderm Cell Types | stem cells
|
|
Endoderm Cell Types
| Endoderm Derived Cell Types
|
| Endoderm Derived Cell Types
|
Exocrine secretory epithelial cells
- Salivary gland mucous cell (polysaccharide-rich secretion)
- Salivary gland number 1 (glycoprotein enzyme-rich secretion)
- Von Ebner's gland cell in tongue (washes taste buds)
- Bowman's gland cell (washes olfactory epithelium)
- Brunner's gland cell in duodenum (enzymes and alkaline mucus)
- Seminal vesicle cell (secretes seminal fluid components, including fructose for swimming Spermatozoon|sperm)
- Prostate gland cell (secretes seminal fluid components)
- Bulbourethral gland cell (mucus secretion)
- Bartholin's gland cell (vaginal lubricant secretion)
- Urethral gland|Gland of Littre cell (mucus secretion)
- Uterus endometrium cell (carbohydrate secretion)
- Insolated goblet cell of respiratory tract|respiratory and Gastrointestinal tract|digestive tracts (mucus secretion)
- Stomach lining mucous cell (mucus secretion)
- Gastric chief cell|Gastric gland zymogenic cell (pepsinogen secretion)
- Parietal cell - Gastric gland oxyntic cell (hydrochloric acid secretion)
- Pancreatic acinar cell (bicarbonate and digestive enzyme secretion
- Paneth cell of small intestine (lysozyme secretion)
- Type II pneumocyte of human lung (surfactant secretion)
- Club cell of lung
|
Hormone-secreting cells
- anterior pituitary cells
- Somatotropes
- Lactotropes
- Thyrotropes
- Gonadotropes
- Corticotropes
- Intermediate pituitary cell, secreting melanocyte-stimulating hormone
- Magnocellular neurosecretory cells
- nonsecreting oxytocin
- secreting vasopressin
- Gut and respiratory tract cells
- secreting serotonin
- secreting endorphin
- secreting somatostatin
- secreting gastrin
- secreting secretin
- nonsecreting cholecystokinin
- secreting insulin
- secreting glucagon
- nonsecreting bombesin
- thyroid gland cells
- thyroid epithelial cell
- Parafollicular cell
- Parathyroid gland cells
- Parathyroid chief cell
- Oxyphil cell (parathyroid)|Oxyphil cell
- Template:Adrenal gland cells
- secreting steroid hormones (mineralocorticoids and gluco corticoids)
- Leydig cell of testes secreting testosterone
- Theca interna cell of ovarian follicle secreting estrogen
- corpus luteum cell of ruptured ovarian follicle secreting progesterone
- Granulosa lutein cells
- Theca lutein cells
- Juxtaglomerular cell (renin secretion)
- Macula densa cell of kidney
- Peripolar cell of kidney
- Mesangial cell of kidney
- Pancreatic islets (islets of Langerhans)
- Alpha cells (secreting glucagon)
- Beta cells (secreting insulin and amylin)
- Delta cells (secreting somatostatin)
- PP cells (gamma cells) (secreting pancreatic polypeptide)
- Epsilon cells (secreting ghrelin)
|
- Links: endoderm | Adult Human Cell Types | Ectoderm Cell Types | Mesoderm Cell Types | Endoderm Cell Types | stem cells
|
|
References
- ↑ Li J, Greco V, Guasch G, Fuchs E & Mombaerts P. (2007). Mice cloned from skin cells. Proc. Natl. Acad. Sci. U.S.A. , 104, 2738-43. PMID: 17299040 DOI.
- ↑ 2.0 2.1 Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R & Anderson DG. (2016). Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. , 22, 306-11. PMID: 26808346 DOI.
- ↑ Strawbridge SE, Clarke J, Guo G & Nichols J. (2022). Deriving Human Naïve Embryonic Stem Cell Lines from Donated Supernumerary Embryos Using Physical Distancing and Signal Inhibition. Methods Mol Biol , 2416, 1-12. PMID: 34870826 DOI.
- ↑ Mehrotra P, Tseropoulos G, Bronner ME & Andreadis ST. (2019). Adult tissue-derived neural crest-like stem cells: Sources, regulatory networks, and translational potential: Concise review. Stem Cells Transl Med , , . PMID: 31738018 DOI.
- ↑ Yamashiro C, Sasaki K, Yabuta Y, Kojima Y, Nakamura T, Okamoto I, Yokobayashi S, Murase Y, Ishikura Y, Shirane K, Sasaki H, Yamamoto T & Saitou M. (2018). Generation of human oogonia from induced pluripotent stem cells in vitro. Science , , . PMID: 30237246 DOI.
- ↑ Dalman A, Totonchi M & Valojerdi MR. (2018). Establishment and characterization of human theca stem cells and their differentiation into theca progenitor cells. J. Cell. Biochem. , , . PMID: 30132968 DOI.
- ↑ Corsinotti A, Wong FC, Tatar T, Szczerbinska I, Halbritter F, Colby D, Gogolok S, Pantier R, Liggat K, Mirfazeli ES, Hall-Ponsele E, Mullin NP, Wilson V & Chambers I. (2017). Distinct SoxB1 networks are required for naïve and primed pluripotency. Elife , 6, . PMID: 29256862 DOI.
- ↑ Nguyen PD, Hollway GE, Sonntag C, Miles LB, Hall TE, Berger S, Fernandez KJ, Gurevich DB, Cole NJ, Alaei S, Ramialison M, Sutherland RL, Polo JM, Lieschke GJ & Currie PD. (2014). Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature , 512, 314-8. PMID: 25119043 DOI.
- ↑ Poh YC, Chen J, Hong Y, Yi H, Zhang S, Chen J, Wu DC, Wang L, Jia Q, Singh R, Yao W, Tan Y, Tajik A, Tanaka TS & Wang N. (2014). Generation of organized germ layers from a single mouse embryonic stem cell. Nat Commun , 5, 4000. PMID: 24873804 DOI.
- ↑ Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S, Tesar PJ, Okada J, Margaretha L, Sperber H, Choi M, Blau CA, Treuting PM, Hawkins RD, Cirulli V & Ruohola-Baker H. (2014). Derivation of naive human embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. , 111, 4484-9. PMID: 24623855 DOI.
- ↑ Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, Masterson K, Larson J, Eaton D, Sadler-Fredd K, Battaglia D, Lee D, Wu D, Jensen J, Patton P, Gokhale S, Stouffer RL, Wolf D & Mitalipov S. (2013). Human embryonic stem cells derived by somatic cell nuclear transfer. Cell , 153, 1228-38. PMID: 23683578 DOI.
- ↑ Nagaoka M, Si-Tayeb K, Akaike T & Duncan SA. (2010). Culture of human pluripotent stem cells using completely defined conditions on a recombinant E-cadherin substratum. BMC Dev. Biol. , 10, 60. PMID: 20525219 DOI.
- ↑ Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP & Daley GQ. (2010). Epigenetic memory in induced pluripotent stem cells. Nature , 467, 285-90. PMID: 20644535 DOI.
- ↑ Garikipati VNS, Singh SP, Mohanram Y, Gupta AK, Kapoor D & Nityanand S. (2018). Isolation and characterization of mesenchymal stem cells from human fetus heart. PLoS ONE , 13, e0192244. PMID: 29420637 DOI.
- ↑ Giritharan G, Ilic D, Gormley M & Krtolica A. (2011). Human embryonic stem cells derived from embryos at different stages of development share similar transcription profiles. PLoS ONE , 6, e26570. PMID: 22039509 DOI.
- ↑ Kanatsu-Shinohara M, Ikawa M, Takehashi M, Ogonuki N, Miki H, Inoue K, Kazuki Y, Lee J, Toyokuni S, Oshimura M, Ogura A & Shinohara T. (2006). Production of knockout mice by random or targeted mutagenesis in spermatogonial stem cells. Proc. Natl. Acad. Sci. U.S.A. , 103, 8018-23. PMID: 16679411 DOI.
- ↑ Ehmcke J, Wistuba J & Schlatt S. (2006). Spermatogonial stem cells: questions, models and perspectives. Hum. Reprod. Update , 12, 275-82. PMID: 16446319 DOI.
- ↑ Aponte PM, van Bragt MP, de Rooij DG & van Pelt AM. (2005). Spermatogonial stem cells: characteristics and experimental possibilities. APMIS , 113, 727-42. PMID: 16480445 DOI.
- ↑ Kanatsu-Shinohara M, Ogonuki N, Iwano T, Lee J, Kazuki Y, Inoue K, Miki H, Takehashi M, Toyokuni S, Shinkai Y, Oshimura M, Ishino F, Ogura A & Shinohara T. (2005). Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development , 132, 4155-63. PMID: 16107472 DOI.
- ↑ Ogawa T, Ohmura M, Yumura Y, Sawada H & Kubota Y. (2003). Expansion of murine spermatogonial stem cells through serial transplantation. Biol. Reprod. , 68, 316-22. PMID: 12493728
- ↑ Fuchs E. (2008). Skin stem cells: rising to the surface. J. Cell Biol. , 180, 273-84. PMID: 18209104 DOI.
- ↑ Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Bühring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, Matzkies M, Reppel M, Hescheler J, Sievert KD, Stenzl A & Skutella T. (2008). Generation of pluripotent stem cells from adult human testis. Nature , 456, 344-9. PMID: 18849962 DOI.
- ↑ Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K & Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell , 131, 861-72. PMID: 18035408 DOI.
- ↑ Hamilton B, Feng Q, Ye M & Welstead GG. (2009). Generation of induced pluripotent stem cells by reprogramming mouse embryonic fibroblasts with a four transcription factor, doxycycline inducible lentiviral transduction system. J Vis Exp , , . PMID: 19915522 DOI.
- ↑ Wakayama S, Ohta H, Hikichi T, Mizutani E, Iwaki T, Kanagawa O & Wakayama T. (2008). Production of healthy cloned mice from bodies frozen at -20 degrees C for 16 years. Proc. Natl. Acad. Sci. U.S.A. , 105, 17318-22. PMID: 18981419 DOI.
- ↑ Thuan NV, Kishigami S & Wakayama T. (2010). How to improve the success rate of mouse cloning technology. J. Reprod. Dev. , 56, 20-30. PMID: 20203432
- ↑ Bourillot PY & Savatier P. (2010). Krüppel-like transcription factors and control of pluripotency. BMC Biol. , 8, 125. PMID: 20875146 DOI.
- ↑ Khan DR, Dubé D, Gall L, Peynot N, Ruffini S, Laffont L, Le Bourhis D, Degrelle S, Jouneau A & Duranthon V. (2012). Expression of pluripotency master regulators during two key developmental transitions: EGA and early lineage specification in the bovine embryo. PLoS ONE , 7, e34110. PMID: 22479535 DOI.
- ↑ Wu R, Xu C, Jin F, Tan Z, Gu B, Chen L, Yao X & Zhang M. (2010). Derivation, characterization and differentiation of a new human embryonic stem cell line from a Chinese hatched blastocyst assisted by a non-contact laser system. Hum. Cell , 23, 89-102. PMID: 20973834 DOI.
- ↑ Hammarberg K & Tinney L. (2006). Deciding the fate of supernumerary frozen embryos: a survey of couples' decisions and the factors influencing their choice. Fertil. Steril. , 86, 86-91. PMID: 16716313 DOI.
- ↑ Hwang WS, Roh SI, Lee BC, Kang SK, Kwon DK, Kim S, Kim SJ, Park SW, Kwon HS, Lee CK, Lee JB, Kim JM, Ahn C, Paek SH, Chang SS, Koo JJ, Yoon HS, Hwang JH, Hwang YY, Park YS, Oh SK, Kim HS, Park JH, Moon SY & Schatten G. (2005). Patient-specific embryonic stem cells derived from human SCNT blastocysts. Science , 308, 1777-83. PMID: 15905366 DOI.
- ↑ Moore KA & Lemischka IR. (2006). Stem cells and their niches. Science , 311, 1880-5. PMID: 16574858 DOI.
Journals
- Cell Stem Cell is the official affiliated journal of the International Society for Stem Cell Research (ISSCR).
- Stem Cells welcomes original articles and concise reviews describing basic laboratory investigations of stem cells and the translation of their clinical aspects of characterization and manipulation from the bench to patient care. The journal covers all aspects of stem cells: embryonic stem cells; tissue-specific stem cells; cancer stem cells; the stem cell niche; stem cell genomics and proteomics; and translational and clinical researc
Reviews
Trounson A & DeWitt ND. (2016). Pluripotent stem cells progressing to the clinic. Nat. Rev. Mol. Cell Biol. , 17, 194-200. PMID: 26908143 DOI.
Mathews DJ, Donovan PJ, Harris J, Lovell-Badge R, Savulescu J & Faden R. (2009). Pluripotent stem cell-derived gametes: truth and (potential) consequences. Cell Stem Cell , 5, 11-4. PMID: 19570509 DOI.
Moore KA & Lemischka IR. (2006). Stem cells and their niches. Science , 311, 1880-5. PMID: 16574858 DOI.
Li L & Xie T. (2005). Stem cell niche: structure and function. Annu. Rev. Cell Dev. Biol. , 21, 605-31. PMID: 16212509 DOI.
Articles
Pekkanen-Mattila M, Pelto-Huikko M, Kujala V, Suuronen R, Skottman H, Aalto-Setälä K & Kerkelä E. (2010). Spatial and temporal expression pattern of germ layer markers during human embryonic stem cell differentiation in embryoid bodies. Histochem. Cell Biol. , 133, 595-606. PMID: 20369364 DOI.
Hiroyama T, Miharada K, Aoki N, Fujioka T, Sudo K, Danjo I, Nagasawa T & Nakamura Y. (2006). Long-lasting in vitro hematopoiesis derived from primate embryonic stem cells. Exp. Hematol. , 34, 760-9. PMID: 16728281 DOI.
Meshorer E & Misteli T. (2006). Chromatin in pluripotent embryonic stem cells and differentiation. Nat. Rev. Mol. Cell Biol. , 7, 540-6. PMID: 16723974 DOI.
Yamazoe H, Kobori M, Murakami Y, Yano K, Satoh M, Mizuseki K, Sasai Y & Iwata H. (2006). One-step induction of neurons from mouse embryonic stem cells in serum-free media containing vitamin B12 and heparin. Cell Transplant , 15, 135-45. PMID: 16719047
Skottman H, Dilber MS & Hovatta O. (2006). The derivation of clinical-grade human embryonic stem cell lines. FEBS Lett. , 580, 2875-8. PMID: 16716780 DOI.
Hammarberg K & Tinney L. (2006). Deciding the fate of supernumerary frozen embryos: a survey of couples' decisions and the factors influencing their choice. Fertil. Steril. , 86, 86-91. PMID: 16716313 DOI.
Moore KA & Lemischka IR. (2006). Stem cells and their niches. Science , 311, 1880-5. PMID: 16574858 DOI.
Search PubMed
May 2006 "stem cell" 154,176 reference articles of which 16,449 were reviews.
Search PubMed Now: stem cell | embryonic stem cell | adult stem cell |
Australia
The Australian Health Ethics Committee was approached by human research ethics committees (HRECs) seeking advice on how to review research protocols that involve stem cell research. The following guidance is interim. Formal guidelines will be developed by AHEC in the context of its review of the 1996 NHMRC Ethical guidelines on assisted reproductive technology.
INFORMATION FOR HUMAN RESEARCH ETHICS COMMITTEES SHEET NUMBER 5 - STEM CELL RESEARCH
USA
- Stem Cells: NIH 2009 Primer | File:NIH Regenerative Medicine 2006.pdf | 2001 Primer | NIH Stem Cell Basics | 2009 NIH Report | Regenerative Medicine 2006 | 2001 NIH Report
National Institute of Health (NIH) Stem Cell Information NIH Stem Cell Basics | NIH Stem Cell Information | NIH Stem Cell Reports | Regenerative Medicine 2006 | Stem Cells: Scientific Progress and Future Research Directions (2001) | National Human Genome Research Institute - Cloning/Embryonic Stem Cells
Stem Cell News (2001)
During the earlier Bush administration there was much political controversy about Stem cells in the USA.
- FDA Letter to Senator Edward M. Kennedy Regarding Stem Cells, September 5, 2001
- Secretary Thompson's Oral Testimony before the Senate Health, Education, Labor and Pensions Committee, September 5, 2001
- National Institutes of Health and WiCell Research Institute, Inc., Sign Stem Cell Research Agreement, September 5, 2001
- National Institutes of Health (NIH) Update on Existing Human Embryonic Stem Cells, August 27, 2001
- Statement by Tommy G. Thompson, Secretary of Health and Human Services, Regarding Stem Cell Lines, August 27, 2001
- Video Broadcast - Briefing by HHS Secretary Tommy G. Thompson on Federal Funding of Human Embryonic Stem Cell Research, August 10, 2001
- NIH Statement on the President's Stem Cell Address, August 9, 2001
- White House Fact Sheet on Embryonic Stem Cell Research, August 9, 2001
- Statement by HHS Secretary Tommy G. Thompson Regarding the President's Decision on Human Embryonic Stem Cell Research, August 9, 2001
- Approval Process for the Documentation of Compliance with the NIH Guidelines on the Use of Human Pluripotent Stem Cells in NIH Research Proposed for Support Under Grants and Cooperative Agreements, November 21, 2000
- Approval Process for the Documentation of Compliance with NIH Guidelines on the Use of Human Pluripotent Stem Cells in NIH Intramural Research, January 16, 2001
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 25) Embryology Stem Cells. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Stem_Cells
- What Links Here?
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G