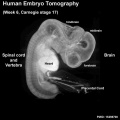
Neural System Development
| Embryology - 26 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Neural development is one of the earliest systems to begin and the last to be completed after birth. This development generates the most complex structure within the embryo and the long time period of development means in utero insult during pregnancy may have consequences to development of the nervous system.
The early central nervous system begins as a simple neural plate that folds to form a neural groove and then neural tube. This early neural is initially open initially at each end forming the neuropores. Failure of these opening to close contributes a major class of neural abnormalities (neural tube defects).
<html5media height="320" width="300">File:Stage 16 MRI 3D03.mp4</html5media>
Within the neural tube stem cells generate the 2 major classes of cells that make the majority of the nervous system : neurons and glia. Both these classes of cells differentiate into many different types generated with highly specialized functions and shapes. This section covers the establishment of neural populations, the inductive influences of surrounding tissues and the sequential generation of neurons establishing the layered structure seen in the brain and spinal cord.
- Neural development beginnings quite early, therefore also look at notes covering Week 3 - neural tube and Week 4 - early nervous system.
- Development of the neural crest and sensory (hearing/vision/smell) are only introduced in these notes and are covered in detail in other notes sections.
Some Recent Findings
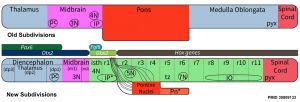 Brain stem subdivisions[1]
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Neural System Development | Neural System Embryology | Neural Tube Closure | Neural Plate | Neuropore | Developmental Myelination | Neural Vascular Development | Cortical Plate |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Objectives
- Understand early neural development.
- Understand the formation of spinal cord.
- Understand the formation of the brain; grey and white matter from the neural tube.
- Understand the role of migration of neurons during neural development.
- To know the main derivatives of the brain vesicles and their walls.
- To know how the nervous system is modelled, cell death etc.
- To understand the contribution of the neural crest.
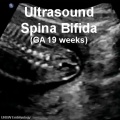
- Understand the developmental basis of certain congenital anomalies of the nervous system, including hydrocephalus, spina bifida, anencephaly and encephalocele.
Reading
UNSW Embryology
The Developing Human: Clinically oriented embryology
Moore, K.L., Persaud, T.V.N. & Torchia, M.G. (2015). The developing human: clinically oriented embryology (10th ed.). Philadelphia: Saunders. (links only function with UNSW connection)

|
|
Larsen's human embryology
Schoenwolf, G.C., Bleyl, S.B., Brauer, P.R., Francis-West, P.H. & Philippa H. (2015). Larsen's human embryology (5th ed.). New York; Edinburgh: Churchill Livingstone. (links only function with UNSW connection)

|
|
Neural Movies
| Neural Development | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
| ||||||||||||||||||
|
|
|
|
|
| ||||||||||||||||||
| Abnormalities Ultrasound | |||||||||||||||||||||||
|
|
|
|
| |||||||||||||||||||
Early Neural Development
The stages below refer to specific Carneigie stages of development (modified from O'Rahilly and Müller 1994[12]).
| Early Neural Timeline | |
|---|---|
| Carnegie Stage | Event |
| 8 | (about 18 postovulatory days) neural groove and folds are first seen |
| 9 | three main divisions of the brain, which are not cerebral vesicles, can be distinguished while the neural groove is still completely open. |
| 10 | (two days later) neural folds begin to fuse near the junction between brain and spinal cord, when neural crest cells are arising mainly from the neural ectoderm |
| 11 | (about 24 days) the rostral (or cephalic) neuropore closes within a few hours; closure is bidirectional, it takes place from the dorsal and terminal lips and may occur in several areas simultaneously. The two lips, however, behave differently. |
| 12 | (about 26 days) The caudal neuropore takes a day to close. The level of final closure is approximately at future somitic pair 31 (corresponds to the level of sacral vertebra 2). Secondary neurulation begins, is the differentiation of the caudal part of the neural tube from the caudal eminence (or end-bud) without the intermediate phase of a neural plate. |
| 13 | (4 weeks) the neural tube is normally completely closed. |
| Links: neural | Week 3 | Week 4 | |
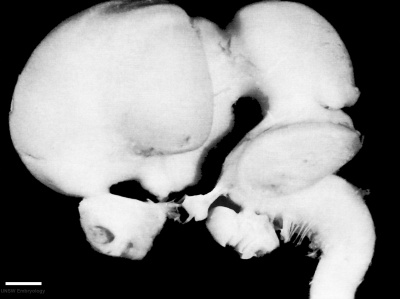
Week 4 to Week 8
| Embryonic Central Nervous System | |||
|---|---|---|---|
| Stage 13 | Stage 14 | Stage 16 | Stage 21 |
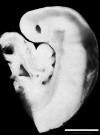
scale bar = 1 mm |
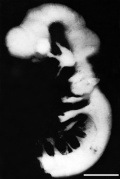
|
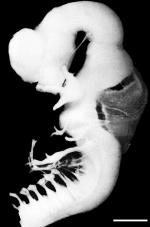
|
|
| Week 4 | Week 5 | Week 6 | Week 8 |
Week 4 - Stage 13
<html5media height="420" width="420">File:Stage 13 MRI 3D02.mp4</html5media>
Week 4 - Stage 16
<html5media height="420" width="420">File:Stage 16 MRI 3D02.mp4</html5media>
Week 8 - Stage 23
<html5media height="500" width="540">File:Stage23 MRI S01.mp4</html5media>
The above MRI scan movie shows the structure of the central nervous system at the end of the embryonic period. Note the relative size and position of the CNS parts, the flexures, the size of the ventricular spaces and chord plexus within this space. There are additional Stage 23 movies available in the links below.
| Stage 23 MRI Movies: Surface | Central Nervous System | CNS (labeled) | Sagittal | Sagittal (labeled) | Transverse | Transverse (labeled) | Coronal | Sagittal Head (labeled) | Sagittal GIT (labeled) | Carnegie stage 23 |
| Week: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Carnegie stage: | 1 2 3 4 | 5 6 | 7 8 9 | 10 11 12 13 | 14 15 | 16 17 | 18 19 | 20 21 22 23 |
| Stage 23 Links: Week 8 | System Development | Lecture - Limb | Lecture - Head Development | Lecture - Sensory | Science Practical - Head | Science Practical - Sensory | Science Practical - Urogenital | Historic - Skull Development | Carnegie Embryos | Madrid Embryos | Category:Carnegie Stage 23 | Next Fetal Development |
| Historic Papers: 1954 Stage 19-23 |
Development Overview
Neuralation begins at the trilaminar embryo with formation of the notochord and somites, both of which underly the ectoderm and do not contribute to the nervous system, but are involved with patterning its initial formation. The central portion of the ectoderm then forms the neural plate that folds to form the neural tube, that will eventually form the entire central nervous system.
- Early developmental sequence: Epiblast - Ectoderm - Neural Plate - Neural groove and Neural Crest - Neural Tube and Neural Crest
| Neural Tube | Primary Vesicles | Secondary Vesicles | Adult Structures |
|---|---|---|---|
| week 3 | week 4 | week 5 | adult |
| prosencephalon (forebrain) | telencephalon | Rhinencephalon, Amygdala, hippocampus, cerebrum (cortex), hypothalamus, pituitary | Basal Ganglia, lateral ventricles | |
| diencephalon | epithalamus, thalamus, Subthalamus, pineal, posterior commissure, pretectum, third ventricle | ||
| mesencephalon (midbrain) | mesencephalon | tectum, Cerebral peduncle, cerebral aqueduct, pons | |
| rhombencephalon (hindbrain) | metencephalon | cerebellum | |
| myelencephalon | medulla oblongata, isthmus | ||
| spinal cord, pyramidal decussation, central canal | |||
Notochord
Does not contribute to the final nervous system, but is critical to patterning the development.
- forms initially as the Axial Process, a hollow tube which extends from the primitive pit , cranially to the oral membrane
- the axial process then allow transient communication between the amnion and the yolk sac through the neuroenteric canal.
- the axial process then merges with the Endodermal layer to form the Notochordal Plate.
- the notochordal plate then rises back into the Mesodermal layer as a solid column of cells which is the Notochord.
- Links: Notochord
Ectoderm
Two main parts with different morphology
- columnar - midline neural plate forming neural tube and neural crest
- cuboidal - lateral surface ectoderm forming epidermis and sensory placodes
- epidermis of skin, hair, glands, anterior pituitary, teeth enamel
- sensory placodes
- Links: Ectoderm
Neural Plate
| The neural plate forms above the notochord and paraxial mesoderm and extends from the buccopharyngeal membrane to primitive node. The cells are described as neuroectodermal and form initially two regions along the head to tail axis: a cranial broad plate region (brain plate) and caudally a narrower plate region (spinal cord).
Neuronal populations are thought to be specified before the plate folds by signals from underlying notochord and mesoderm, as well as signals spread laterally through the plate.
| |||
|
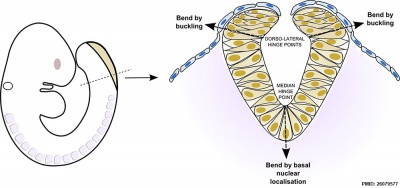
Neural Bending
There are two bending processes occurring in the formation of the neural groove and neural tube.
- occuring in the midline due to cells in this region having a basal nuclear localisation. This initial bending leads to formation of the neural groove.
- occuring at the dorsolateral hinge points by different mechanism involving "buckling". This later bending leads to formation of the neural tube.
Mouse neural tube bending model (see review[13])
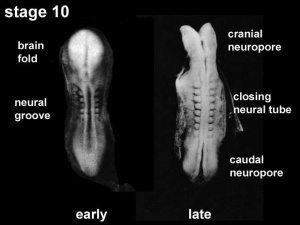
Neural Groove
|
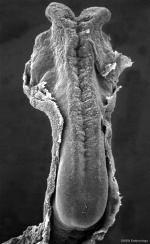
In the human embryo the neural groove forms in the midline of the neural plate (day 18-19).
|
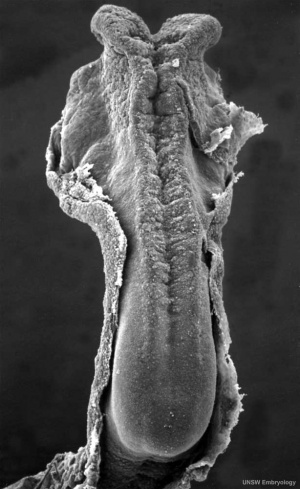
Neural Groove human embryo (Carnegie stage 10, week 4) |
Neural Tube
- fusion of neural groove extends rostrally and caudally
- begins at level of 4th somite, "zips up" neural groove
- leaves 2 openings at either end- Neuropores
- forms the brain and spinal cord
- Secondary Neuralation - caudal end of neural tube formed by secondary neuralation, develops from primitive streak region, solid cord canalized by extension of neural canal. mesodermal caudal eminence
Neuropores
| Cranial Neuropore | Caudal Neuropore |
|---|---|
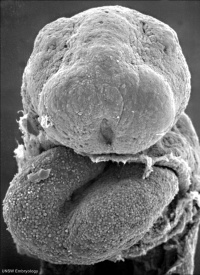
|
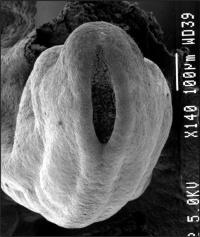
|
| (stage 11) | (stage 12) |
- cranial (anterior) neuropore closes before caudal (posterior)
- failure to close - Neural Tube Defects (NTD), severity dependent upon level, spina bifida anancephaly (More? [neuron2.htm Neural Abnormalities])
- found that supplementation of maternal diet with folate reduces incidence of NTDs
- A randomised controlled trial conducted by the Medical Research Council of the United Kingdom demonstrated a 72% reduction in risk of recurrence by periconceptional (ie before and after conception) folic acid supplementation (4mg daily).
- Women who have one infant with a neural tube defect have a significantly increased risk of recurrence (40-50 per thousand compared with 2 per thousand for all births)
Lamina Terminalis
| Note the site of the embryonic cranial neuropore can later be identified within the central nervous system as the lamina terminalis. | 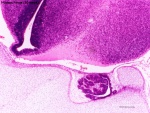
Human Fetus (week 10) brain showing lamina terminalis region |
Neural Crest
- a population of cells at the edge of the neural plate that lie dorsally when the neural tube fuses
- dorsal to the neural tube, as a pair of streaks
- cells migrate throughout the embryo
- studied by quail-chick chimeras - transplanted quail cells have obvious nucleoli compared with chicken Neural Crest Derivitives
- pluripotential, forms many different types of cells: dorsal root ganglia (neurons, sheath cells, glia), autonomic ganglia, adrenal medulla, pia-arachnoid sheath, skin melanocytes, connective tissue of cardiac outflow, thyroid parafollicular cells, craniofacial skeleton and teeth odontoblasts.
- Links: Neural Crest Development
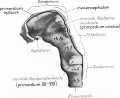
Early Brain Structure
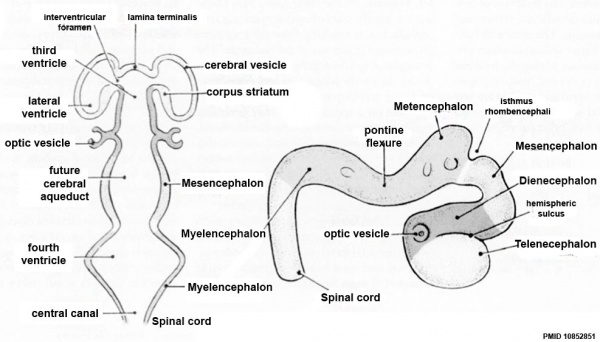
Primary Vesicles
- rostral neural tube forms 3 primary brain vesicles (week 4)
- 3 primary vesicles: prosencephalon (forebrain), mesencephalon (midbrain), rhombencephalon (hindbrain)
See also the week 4 embryo movie.
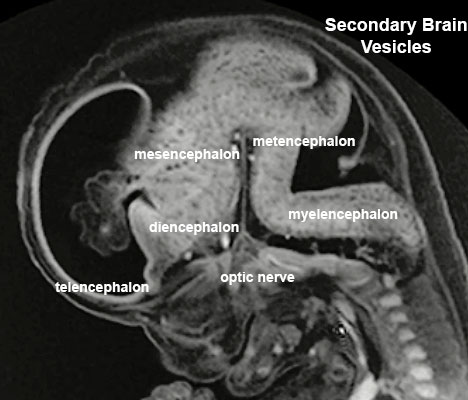
Secondary Vesicles
From the 3 primary vesicles developing to form 5 secondary vesicles (week 5)
- prosencephalon- telencephalon (endbrain, forms cerebral hemispheres), diencephalon (betweenbrain, forms optic outgrowth)
- mesencephalon
- rhombencephalon- metencephalon (behindbrain), myelencephalon (medullabrain)
See also the week 6 embryo movie.
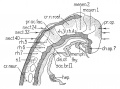
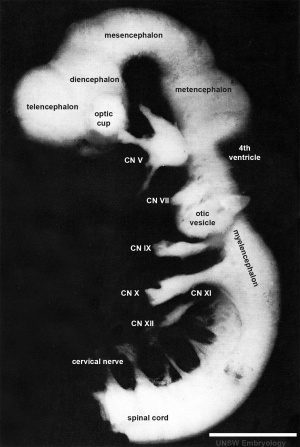
| Carnegie Stage 14 Secondary Vesicles (Week 5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lateral view of the central nervous system of embryo at Carnegie stage 14 (Scale bar is 1 mm). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Week 8 - Stage 23
Ventricles
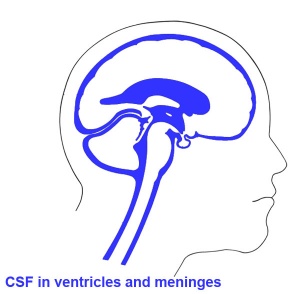
- cavity within tube will form the contiguious space of the ventricules of the brain and central canal of spinal cord
- this space is filled initially with amniotic fluid, later with CerebroSpinal Fluid (CSF)
- CSF is secreted by a modified vascular structure, the chorioid plexus, lying within the ventricles
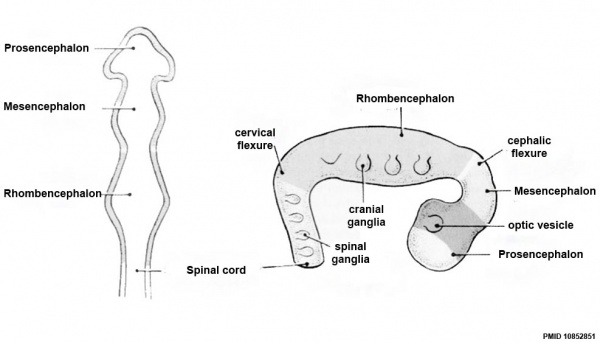
Brain Flexures
Rapid growth folds the neural tube forming 3 brain flexures (cranial to caudal)
- cephalic flexure - (mesencephalic) pushes mesencephalon upwards
- pontine flexure - (metencephalon) generates 4th ventricle
- cervical flexure - (myelencephalon) between brain stem and spinal cord
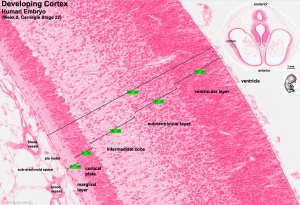
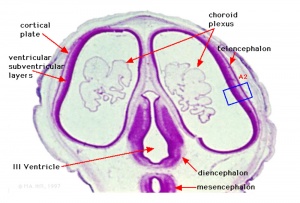
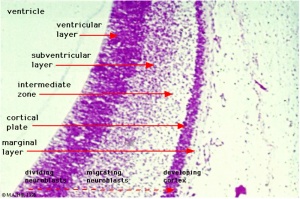
Neural Layers
- neural stem cells lie in the layer closest to the ventricular space, the ventricular layer
- this layer generates both neuroblasts and glioblasts
Neuroblasts - neurons arise first as neuroblasts and migrate along radial gial, their migration stops at cortical plate. Glioblasts - glia arise later as glioblasts
Both neurons and glia undergo a complex process of growth, differentiation and interaction over a long developmental time period.
Spinal Cord Axes
- Experimental manipulation of interactions.
- Initial experiments looked at how isolated tissues may influence the development of the spinal cord.
- Repositionining of specific tissues both in vivo and in vitro
- specific markers of or alteration of differentiation.
Notocord Induction
- Ventral- Sonic Hedgehog
- notochord secretes sonic hedgehog
- Gene expression studies (ISH) showed shh gene expression occured in a subset of inducing tissues
- has a patterning role elsewhere (limb, sclerotome, lung)
- 2 signaling activities acting (locally and at a distance) Ventral- Sonic Hedgehog
- Binds to cell surface receptor patched
- without shh, patched (Ptc) binds smoothened (Smo)
- with shh shh-Ptc releases Smo activating G protein pathway
Early Development and Neural Derivatives
- bilaminar embryo- hyoblast
- trilaminar embryo then ectoderm layer, neural plate, neural groove, neural tube and neural crest
- cranial expansion of neural tube- central nervous system
- caudal remainder of neural tube- spinal cord
- neural crest
- dorsal root ganglia
- parasympathetic / sympathetic ganglia.
- ectodermal placodes- components of the special senses: otic placode (otocyst), nasal placode, lens placode
Links: Placodes
Neural tube and Genes: neural specification- Notch/Delta, patched receptor. Border- fibroblast growth factor (fgf), BMP (BMP4, msx1) Rostral border- Dlx5
Neural Tube Patterning
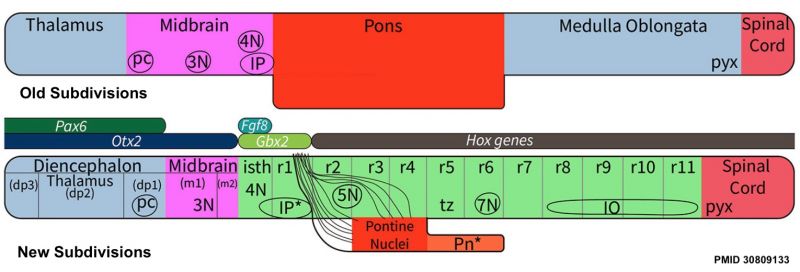
Review - Time for Radical Changes in Brain Stem Nomenclature-Applying the Lessons From Developmental Gene Patterns[1] "The traditional subdivision of the brain stem into midbrain, pons, and medulla oblongata is based purely on the external appearance of the human brain stem. There is an urgent need to update the names of brain stem structures to be consistent with the discovery of rhomobomeric segmentation based on gene expression. The most important mistakes are the belief that the pons occupies the upper half of the hindbrain, the failure to recognize the isthmus as the first segment of the hindbrain, and the mistaken inclusion of diencephalic structures in the midbrain. The new nomenclature will apply to all mammals. This essay recommends a new brain stem nomenclature based on developmental gene expression, progeny analysis, and fate mapping."
Molecular Patterning Molecules
- segmented along its length - Hox/Lim gene expression
- ventral identity - sonic hedgehog, BMP7/chordin interaction
- dorsal identity - dorsalin
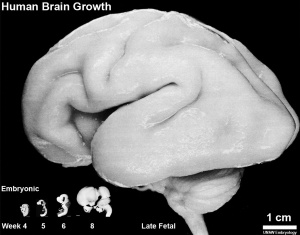
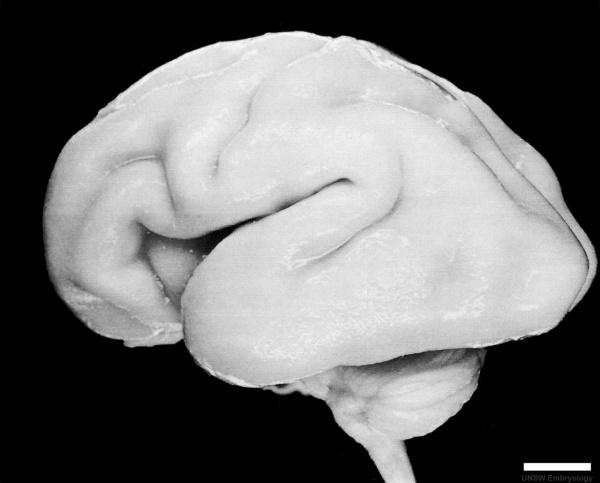
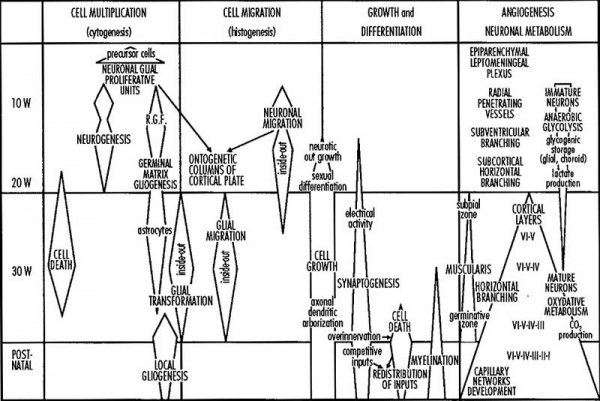
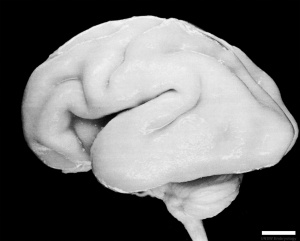
Fetal Development
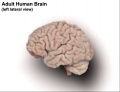
Human Fetus (CRL 240mm) Brain (left dorsolateral view)
For more details see Neural System - Fetal
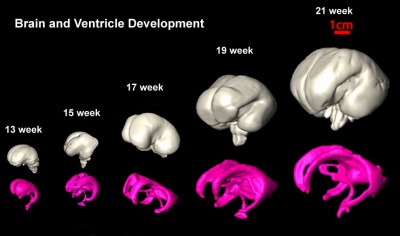
Fetal - Second Trimester
|
|
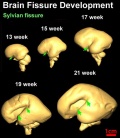
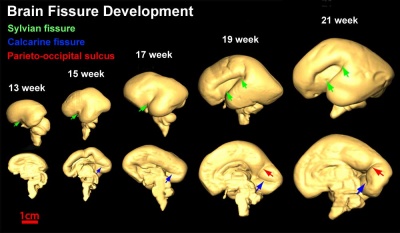
| Brain and Ventricular Development[14] | Brain Fissure Development[14] |
Third Trimester
Three-dimensional magnetic resonance imaging and image-processing algorithms have been used to quantitate between 29-41 weeks volumes of: total brain, cerebral gray matter, unmyelinated white matter, myelinated, and cerebrospinal fluid (grey matter- mainly neuronal cell bodies; white matter- mainly neural processes and glia). A study of 78 premature and mature newborns showed that total brain tissue volume increased linearly over this period at a rate of 22 ml/week. Total grey matter also showed a linear increase in relative intracranial volume of approximately 1.4% or 15 ml/week. The rapid increase in total grey matter is mainly due to a fourfold increase in cortical grey matter. Quantification of extracerebral and intraventricular CSF was found to change only minimally.[15]
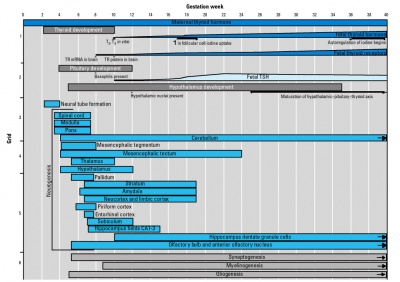
Thyroid System and Neural Development
Human thyroid system and neural development
Timeline of human thyroid system and brain development from conception to birth.[16] (Estimation of neurogenesis adapted from Bayer et al.[17])
- Links: thyroid
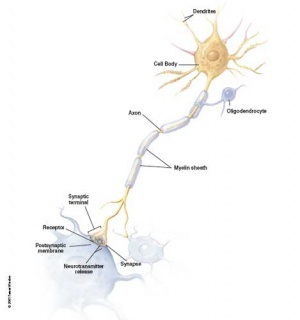
Gliogenesis and Myelination
Glial cells have many different types and roles in central and peripheral neural development, though historically described as "supportive". (More? gliogenesis and myelination) These central glia develop from the same neural stem cells as neurons, while peripheral glia (Schwann cells) are derived from neural crest.
Early in neural development a special type of developmental glia, radial glia, provide pathway for developing neuron (neuroblasts) migration out from the proliferating ventricular layer and are involved in the subsequent lamination and columnar organization of the central nervous system.
Types of glia: radial glia, astroglia, oligodendroglia, microglia and Schwann cells.
- Links: gliogenesis | Schwann cell
Gene Diseases - Sonic Hedgehog
SHH Human mutation- holoprosencephaly 3
- characteristic facies of the severe form of HPE which included a single fused eye (cyclopia) and a nose-like structure (proboscis) above the eye
- Downstream targets of Sonic hedgehog signalling: transcription factors like Gli3 (responsible for Greigs polycephalosyndactyly in humans), d Hoxd13 (responsible for polysyndactyly)
References
- ↑ 1.0 1.1 1.2 Watson C, Bartholomaeus C & Puelles L. (2019). Time for Radical Changes in Brain Stem Nomenclature-Applying the Lessons From Developmental Gene Patterns. Front Neuroanat , 13, 10. PMID: 30809133 DOI.
- ↑ Micali N, Kim SK, Diaz-Bustamante M, Stein-O'Brien G, Seo S, Shin JH, Rash BG, Ma S, Wang Y, Olivares NA, Arellano JI, Maynard KR, Fertig EJ, Cross AJ, Bürli RW, Brandon NJ, Weinberger DR, Chenoweth JG, Hoeppner DJ, Sestan N, Rakic P, Colantuoni C & McKay RD. (2020). Variation of Human Neural Stem Cells Generating Organizer States In Vitro before Committing to Cortical Excitatory or Inhibitory Neuronal Fates. Cell Rep , 31, 107599. PMID: 32375049 DOI.
- ↑ Turk E, van den Heuvel MI, Benders MJ, de Heus R, Franx A, Manning JH, Hect JL, Hernandez-Andrade E, Hassan SS, Romero R, Kahn RS, Thomason ME & van den Heuvel MP. (2019). Functional connectome of the fetal brain. J. Neurosci. , , . PMID: 31685648 DOI.
- ↑ Metzis V, Steinhauser S, Pakanavicius E, Gouti M, Stamataki D, Ivanovitch K, Watson T, Rayon T, Mousavy Gharavy SN, Lovell-Badge R, Luscombe NM & Briscoe J. (2018). Nervous System Regionalization Entails Axial Allocation before Neural Differentiation. Cell , , . PMID: 30343898 DOI.
- ↑ Sousa AMM, Zhu Y, Raghanti MA, Kitchen RR, Onorati M, Tebbenkamp ATN, Stutz B, Meyer KA, Li M, Kawasawa YI, Liu F, Perez RG, Mele M, Carvalho T, Skarica M, Gulden FO, Pletikos M, Shibata A, Stephenson AR, Edler MK, Ely JJ, Elsworth JD, Horvath TL, Hof PR, Hyde TM, Kleinman JE, Weinberger DR, Reimers M, Lifton RP, Mane SM, Noonan JP, State MW, Lein ES, Knowles JA, Marques-Bonet T, Sherwood CC, Gerstein MB & Sestan N. (2017). Molecular and cellular reorganization of neural circuits in the human lineage. Science , 358, 1027-1032. PMID: 29170230 DOI.
- ↑ Sousa AMM, Meyer KA, Santpere G, Gulden FO & Sestan N. (2017). Evolution of the Human Nervous System Function, Structure, and Development. Cell , 170, 226-247. PMID: 28708995 DOI.
- ↑ Yang HJ, Lee DH, Lee YJ, Chi JG, Lee JY, Phi JH, Kim SK, Cho BK & Wang KC. (2014). Secondary neurulation of human embryos: morphological changes and the expression of neuronal antigens. Childs Nerv Syst , 30, 73-82. PMID: 23760472 DOI.
- ↑ Ozair MZ, Kintner C & Brivanlou AH. (2013). Neural induction and early patterning in vertebrates. Wiley Interdiscip Rev Dev Biol , 2, 479-98. PMID: 24014419 DOI.
- ↑ Ponti G, Obernier K, Guinto C, Jose L, Bonfanti L & Alvarez-Buylla A. (2013). Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc. Natl. Acad. Sci. U.S.A. , 110, E1045-54. PMID: 23431204 DOI.
- ↑ Pyrgaki C, Trainor P, Hadjantonakis AK & Niswander L. (2010). Dynamic imaging of mammalian neural tube closure. Dev. Biol. , 344, 941-7. PMID: 20558153 DOI.
- ↑ Massa V, Savery D, Ybot-Gonzalez P, Ferraro E, Rongvaux A, Cecconi F, Flavell R, Greene ND & Copp AJ. (2009). Apoptosis is not required for mammalian neural tube closure. Proc. Natl. Acad. Sci. U.S.A. , 106, 8233-8. PMID: 19420217 DOI.
- ↑ O'Rahilly R & Müller F. (1994). Neurulation in the normal human embryo. Ciba Found. Symp. , 181, 70-82; discussion 82-9. PMID: 8005032
- ↑ McShane SG, Molè MA, Savery D, Greene ND, Tam PP & Copp AJ. (2015). Cellular basis of neuroepithelial bending during mouse spinal neural tube closure. Dev. Biol. , 404, 113-24. PMID: 26079577 DOI.
- ↑ 14.0 14.1 Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, Miller MI & Mori S. (2009). Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J. Neurosci. , 29, 4263-73. PMID: 19339620 DOI.
- ↑ Hüppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, Tsuji MK & Volpe JJ. (1998). Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. , 43, 224-35. PMID: 9485064 DOI.
- ↑ Howdeshell KL. (2002). A model of the development of the brain as a construct of the thyroid system. Environ. Health Perspect. , 110 Suppl 3, 337-48. PMID: 12060827
- ↑ Bayer SA, Altman J, Russo RJ & Zhang X. (1993). Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology , 14, 83-144. PMID: 8361683
Journals
- Neural Development Browse contents
- Developmental Brain Research Content Listing
- Neural Development Welcome to Neural Development | Pubmed Central Volume 1 2006 | Pubmed Central Volume 2 2007 |
- International Journal for Developmental Neuroscience Official Journal of the International Society for Developmental Neuroscience |
- Developmental Neuroscience Journal Homepage | Hippocampal Development | Vol. 29, No. 3, 2007 |
- Neuroscience Official journal of The International Brain Research Organisation (IBRO)
- Neuron Neuroscience journal published by Cell press
Online Textbooks
Developmental Biology (6th ed) Gilbert, Scott F. Sunderland (MA): Sinauer Associates, Inc.; c2000. Formation of the Neural Tube | Differentiation of the Neural Tube | Tissue Architecture of the Central Nervous System | Neuronal Types | Snapshot Summary: Central Nervous System and Epidermis
Neuroscience Purves, Dale; Augustine, George J.; Fitzpatrick, David; Katz, Lawrence C.; LaMantia, Anthony-Samuel; McNamara, James O.; Williams, S. Mark. Sunderland (MA): Sinauer Associates, Inc. ; c2001 Early Brain Development | Construction of Neural Circuits | Modification of Brain Circuits as a Result of Experience
Molecular Biology of the Cell (4th Edn) Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter. New York: Garland Publishing; 2002. Neural Development | The three phases of neural development
Health Services/Technology Assessment Text (HSTAT) Bethesda (MD): National Library of Medicine (US), 2003 Oct. Developmental Disorders Associated with Failure to Thrive
Search NLM Online Textbooks- "neural development" : Developmental Biology | The Cell- A molecular Approach | Molecular Biology of the Cell | Endocrinology
Reviews
Baker NE & Brown NL. (2018). All in the family: proneural bHLH genes and neuronal diversity. Development , 145, . PMID: 29720483 DOI.
Miranda A & Sousa N. (2018). Maternal hormonal milieu influence on fetal brain development. Brain Behav , 8, e00920. PMID: 29484271 DOI.
Nikolopoulou E, Galea GL, Rolo A, Greene ND & Copp AJ. (2017). Neural tube closure: cellular, molecular and biomechanical mechanisms. Development , 144, 552-566. PMID: 28196803 DOI.
Hardwick LJ, Ali FR, Azzarelli R & Philpott A. (2015). Cell cycle regulation of proliferation versus differentiation in the central nervous system. Cell Tissue Res. , 359, 187-200. PMID: 24859217 DOI.
Götz M & Huttner WB. (2005). The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. , 6, 777-88. PMID: 16314867 DOI.
Greene ND & Copp AJ. (2009). Development of the vertebrate central nervous system: formation of the neural tube. Prenat. Diagn. , 29, 303-11. PMID: 19206138 DOI.
Articles
Shinotsuka N, Yamaguchi Y, Nakazato K, Matsumoto Y, Mochizuki A & Miura M. (2018). Caspases and matrix metalloproteases facilitate collective behavior of non-neural ectoderm after hindbrain neuropore closure. BMC Dev. Biol. , 18, 17. PMID: 30064364 DOI.
Nikolopoulou E, Galea GL, Rolo A, Greene ND & Copp AJ. (2017). Neural tube closure: cellular, molecular and biomechanical mechanisms. Development , 144, 552-566. PMID: 28196803 DOI.
Yang HJ, Lee DH, Lee YJ, Chi JG, Lee JY, Phi JH, Kim SK, Cho BK & Wang KC. (2014). Secondary neurulation of human embryos: morphological changes and the expression of neuronal antigens. Childs Nerv Syst , 30, 73-82. PMID: 23760472 DOI.
Saitsu H & Shiota K. (2008). Involvement of the axially condensed tail bud mesenchyme in normal and abnormal human posterior neural tube development. Congenit Anom (Kyoto) , 48, 1-6. PMID: 18230116 DOI.
. (1970). Embryonic vertebrate central nervous system: revised terminology. The Boulder Committee. Anat. Rec. , 166, 257-61. PMID: 5414696 DOI.
Search PubMed
Search Pubmed: Neural System Development | Neural Development | Neural Tube Development | Spinal Cord Development
NCBI - Policies and Guidelines | PubMed | Help:Reference Tutorial
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
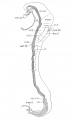
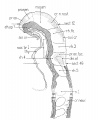
Bartelmez GW. The subdivisions of the neural folds in man. (1923) J. Comp. Neural., 35: 231-247.
fig 3 Eternod’s ‘DuGa’ embryo Carnegie stage 10 Week 4
fig 4 Veit and Esch embryo Carnegie stage 10 Week 4
fig 5 Carnegie Collection Embryo no. 1201a. Carnegie stage 11 Week 4
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Terms
| Neural Terms |
|---|
Neural Development
|
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
| System Links: Introduction | Cardiovascular | Coelomic Cavity | Endocrine | Gastrointestinal Tract | Genital | Head | Immune | Integumentary | Musculoskeletal | Neural | Neural Crest | Placenta | Renal | Respiratory | Sensory | Birth |
Cite this page: Hill, M.A. (2024, April 26) Embryology Neural System Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_System_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G