Pig Development
| Embryology - 26 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Pig (Sus scrofa) developmental model is studied extensively due to the commercial applications of pigs for meat production and for health issues such as obesity, cardiovascular disease, and organ transplantation (xenotransplantation).
Historically, there is an excellent description of the pig reproductive estrous cycle and the cyclic changes that occur within the ovary.[1]
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Pig Embryology | Pig Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Taxon
Taxonomy ID: 9823
Genbank common name: pig
Inherited blast name: even-toed ungulates
Rank: species
Genetic code: Translation table 1 (Standard)
Mitochondrial genetic code: Translation table 2 (Vertebrate Mitochondrial)
Other names: wild boar, swine, pigs
Lineage (full): cellular organisms; Eukaryota; Fungi/Metazoa group; Metazoa; Eumetazoa; Bilateria; Coelomata; Deuterostomia; Chordata; Craniata; Vertebrata; Gnathostomata; Teleostomi; Euteleostomi; Sarcopterygii; Tetrapoda; Amniota; Mammalia; Theria; Eutheria; Laurasiatheria; Cetartiodactyla; Suina; Suidae; Sus
Animal Models
| Postnatal Animal Models | mouse | rat | pig |
|---|---|---|---|
| Pregnancy period (days) | 18 – 21 | 21 – 23 | 110 – 118 |
| Placenta type | Discoidal, decidual hemoendothelial choroidea |
Discoidal, decidual hemoendothelial choroidea |
Epitheliochorial |
| Litter size | 6 – 12 | 6 – 15 | 11 – 16 |
| Birth weight (g) | 0.5 – 1.5 | 3 – 5 | 900 – 1600 |
| Weaning weight male/female (g) | 18 – 25/16 – 25 | 55 – 90/45 – 80 | 6000 – 8000 |
| Suckling period (days) | 21–28 | 21 | 28–49 |
| Solid diet beginning (days) | 10 | 12 | 12 – 15 |
| Puberty male/female (week) | 4 – 6/5 | 6/6 – 8 | 20 – 28 |
| Life expectancy (years) | 1 - 2 | 2 - 3 | 14 – 18 |
| Table data - Otis and Brent (1954)[8] Links: timeline | |||
Carnegie Stages Comparison Table
| Species | Stage | |||||||||||||||
| Human | Days | 20 | 22 | 24 | 28 | 30 | 33 | 36 | 40 | 42 | 44 | 48 | 52 | 54 | 55 | 58 |
| Pig | Days | 14 | 15 | 16 | 17 | 18 | 19 | 20.5 | 21.5 | 23 | 24 | 25.5 | 27.5 | 29 | 30.5 | 32.5 |
Table data - Otis and Brent (1954)[8]
- Links: Carnegie Stage Comparison
Reproductive Overview
- The gestation period of a pig is 112 to 114 days.
- Female pigs can become pregnant at around 8 to 18 months of age.
- The pig has an estrus cycle occurring every 21 days if not bred.
- Male pigs become sexually active at 8 to 10 months of age.
- Embryos begin to attach to the uterus on days 13–14 of pregnancy.
- Day 15-20 implanted and expansion of allantois.
- A litter of piglets is between 6 and 12 piglets.
Normal Stages
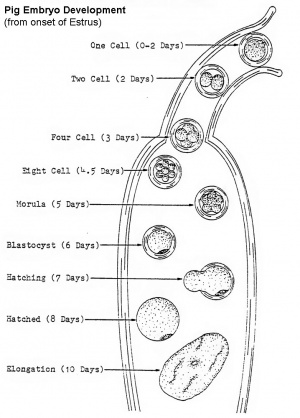
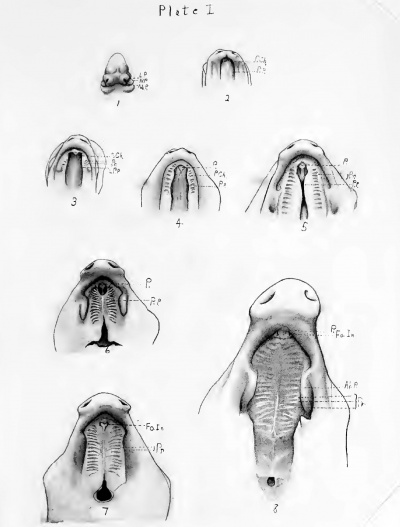
The images below are from the 1897 Normentafeln zur Entwicklungsgeschichte der Wirbeltiere - Sus scrofa domesticus (Normal Plates of the Development of the Pig Embryo) by Franz Keibel
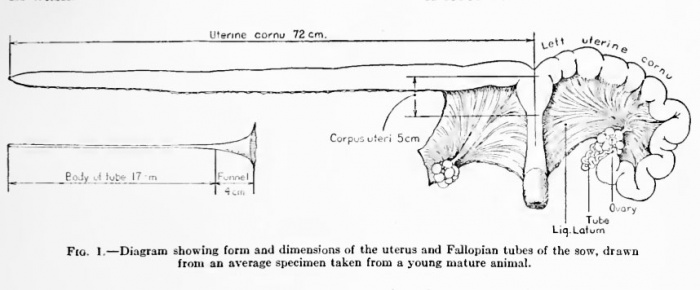
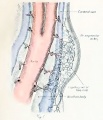
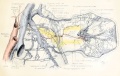
Uterus and Ovary
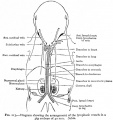
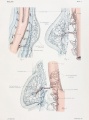
Diagram showing form and dimensions of the uterus and Fallopian tubes of the sow.[1] Drawn from an average specimen taken from a young mature animal.
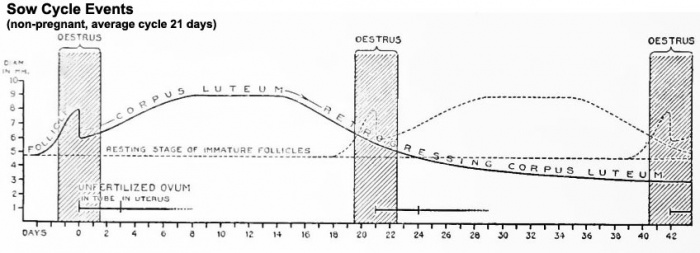
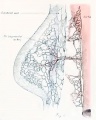
Estrous Cycle
Female pig is called a sow.
Non-Pregnant
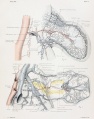
Events of the average cycle of 21 days in the non-pregnant sow.[1]
Diagram showing relationship between oestrua, ovulation, corpus luteum development, and the progress of the ova in the sow.
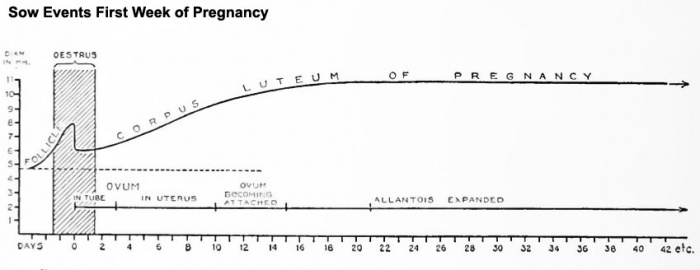
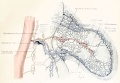
Pregnant
Events of the first weeks of pregnancy.[1]
Diagram showing relationship between oestrua, ovulation, corpus luteum development, and the progress of the ova in the sow.
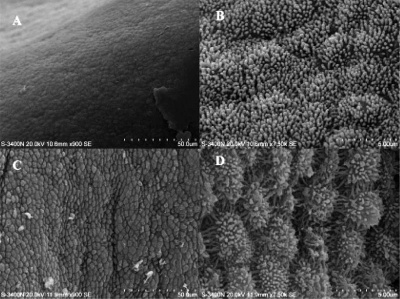
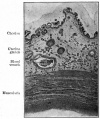
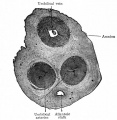
Scanning electron microscope images of the endometrial surface of a Day 13 pregnant sow.[9]
Male Pig
Male pig is called a boar.
Spermatozoa
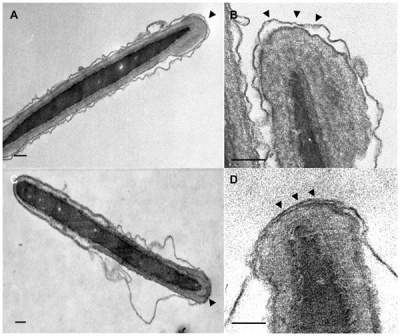
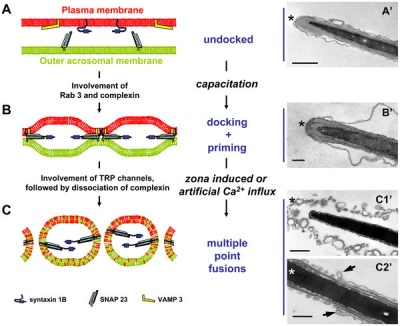
Capacitation alters the ultrastructure of the apical head and the acrosome of boar sperm.[6]
Model for capacitation-induced stable docking of the acrosome to the sperm plasma membrane.[6]
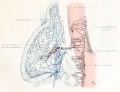
Testis
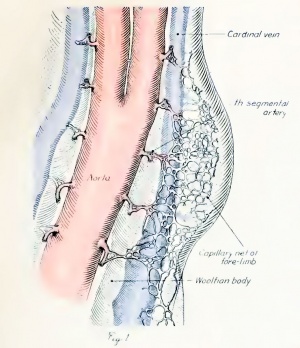
The testis of the pig receives its first blood supply when the embryo is 33 mm in length.[10]
Neural Development
The data below is summarised from an excellent study of early neural development in the pig.[11] The same authors have studied neural development in the rabbit.
- 7 somite embryo - first apposition of the neural folds occurs at somite levels 5-7. (corresponds to closure site I in mouse).
- next stage - rostral and caudal parts of the rhombencephalic folds appose, leaving an opening in between.
- at this stage four neuropores can be distinguished, of which the anterior and posterior ones will remain open longest. (two rhombencephalic closure sites have no counterpart in the mouse, but do have some resemblance to those of the rabbit)
anterior neuropore
- closes in three phases
- dorsal folds slowly align and then close instantaneously, the slow progression being likely due to a counteracting effect of the mesencephalic flexure
- dorso-lateral folds close in a zipper-like fashion in caudo-rostral direction
- final round aperture is likely to close by circumferential growth.
22 somite embryo - anterior neuropore is completely closed. (closure sites for the anterior neuropore in mouse embryo, none of these were detected in the pig embryo)
posterior neuropore
- closes initially very fast in the somitic region, but this process almost stops thereafter.
- stage 20-22 somites the posterior neuropore suddenly reduces in size but thereafter a small neuropore remains for 5 somite stages.
- closure of the posterior neuropore is completed at the stage of 28 somites.
8-20 somite embryos - the width of the posterior neuropore does not change, while the rate of closure gradually increases.
Palate Development
Plates below are from a 1916 thesis on palate development in the pig.[12]
Miniature Pig Palate Timeline[2]
- E30 - growth of the bilateral palatal shelves alongside the tongue
- E35 - elevation of the horizontal position above the tongue
- E35-50 - establishment of bilateral shelf contact at the midline
- E50 - final palate fusion step
Additional Images
References
- ↑ 1.0 1.1 1.2 1.3 Corner GW. Abnormalities of the mammalian embryo occurring before implantation. (1922) Contrib. Embryol., Carnegie Inst. Wash. Publ. 60, : 61-66.
- ↑ 2.0 2.1 Sun L, Wang J, Liu H, Fan Z, Wang S & Du J. (2017). A Comprehensive Study of Palate Development in Miniature Pig. Anat Rec (Hoboken) , 300, 1409-1419. PMID: 28296336 DOI.
- ↑ Xue B, Li Y, He Y, Wei R, Sun R, Yin Z, Bou G & Liu Z. (2016). Porcine Pluripotent Stem Cells Derived from IVF Embryos Contribute to Chimeric Development In Vivo. PLoS ONE , 11, e0151737. PMID: 26991423 DOI.
- ↑ Conrad MS, Sutton BP, Dilger RN & Johnson RW. (2014). An in vivo three-dimensional magnetic resonance imaging-based averaged brain collection of the neonatal piglet (Sus scrofa). PLoS ONE , 9, e107650. PMID: 25254955 DOI.
- ↑ Freeman TC, Ivens A, Baillie JK, Beraldi D, Barnett MW, Dorward D, Downing A, Fairbairn L, Kapetanovic R, Raza S, Tomoiu A, Alberio R, Wu C, Su AI, Summers KM, Tuggle CK, Archibald AL & Hume DA. (2012). A gene expression atlas of the domestic pig. BMC Biol. , 10, 90. PMID: 23153189 DOI.
- ↑ 6.0 6.1 6.2 Tsai PS, Garcia-Gil N, van Haeften T & Gadella BM. (2010). How pig sperm prepares to fertilize: stable acrosome docking to the plasma membrane. PLoS ONE , 5, e11204. PMID: 20585455 DOI.
- ↑ Hassoun R, Schwartz P, Feistel K, Blum M & Viebahn C. (2009). Axial differentiation and early gastrulation stages of the pig embryo. Differentiation , 78, 301-11. PMID: 19683851 DOI.
- ↑ 8.0 8.1 Otis EM and Brent R. Equivalent ages in mouse and human embryos. (1954) Anat Rec. 120(1):33-63. PMID 13207763
- ↑ Ren Q, Guan S, Fu J & Wang A. (2010). Temporal and spatial expression of Muc1 during implantation in sows. Int J Mol Sci , 11, 2322-35. PMID: 20640155 DOI.
- ↑ Hill EC. Development and vascularization of the testis. (1906) Amer. J Anat. 6: 390-360.
- ↑ van Straaten HW, Peeters MC, Hekking JW & van der Lende T. (2000). Neurulation in the pig embryo. Anat. Embryol. , 202, 75-84. PMID: 10985427
- ↑ Baumgartner RA. Development of the palate and the definitive choanae in the pig. (1916) Thesis, University of Illinois.
Reviews
Buddington RK, Sangild PT, Hance B, Huang EY & Black DD. (2012). Prenatal gastrointestinal development in the pig and responses after preterm birth. J. Anim. Sci. , 90 Suppl 4, 290-8. PMID: 23365359 DOI.
Somfai T, Kikuchi K & Nagai T. (2012). Factors affecting cryopreservation of porcine oocytes. J. Reprod. Dev. , 58, 17-24. PMID: 22450280
Ostrup E, Hyttel P & Ostrup O. (2011). Embryo-maternal communication: signalling before and during placentation in cattle and pig. Reprod. Fertil. Dev. , 23, 964-75. PMID: 22127002 DOI.
Waclawik A. (2011). Novel insights into the mechanisms of pregnancy establishment: regulation of prostaglandin synthesis and signaling in the pig. Reproduction , 142, 389-99. PMID: 21677026 DOI.
Robison OW. (1976). Growth patterns in swine. J. Anim. Sci. , 42, 1024-35. PMID: 770410
Book SA & Bustad LK. (1974). The fetal and neonatal pig in biomedical research. J. Anim. Sci. , 38, 997-1002. PMID: 4596894
Moor RM. (1968). Foetal homeostasis: conceptus-ovary endocrine balance. Proc. R. Soc. Med. , 61, 1217-26. PMID: 4973146
Moor RM. (1968). Effect of embryo on corpus luteum function. J. Anim. Sci. , 27 Suppl 1, 97-118. PMID: 4951167
Articles
Zhang L, Lin Z, Bi Y, Zheng X, Xiao H & Hua Z. (2018). CO2 concentration affects in vitro pig embryo developmental capacity. Pol J Vet Sci , 21, 609-614. PMID: 30468346 DOI.
Liu J, Zhu Y, Luo GZ, Wang X, Yue Y, Wang X, Zong X, Chen K, Yin H, Fu Y, Han D, Wang Y, Chen D & He C. (2016). Abundant DNA 6mA methylation during early embryogenesis of zebrafish and pig. Nat Commun , 7, 13052. PMID: 27713410 DOI.
Hassoun R, Schwartz P, Rath D, Viebahn C & Männer J. (2010). Germ layer differentiation during early hindgut and cloaca formation in rabbit and pig embryos. J. Anat. , 217, 665-78. PMID: 20874819 DOI.
Search PubMed
Search Pubmed: pig development | pig embryo | Sus scrofa development
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- NCBI - Pig Genome
- USA - PigBase a computer database that includes information on papers published about gene mapping in the pig.
- NSW Agriculture - Pig breeds and breeding
- AGBU - Pig Genetics
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 26) Embryology Pig Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Pig_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G